The document summarizes the hexose monophosphate pathway (HMP pathway), also known as the pentose phosphate pathway (PPP). The key points are:
1. The HMP pathway is an alternative glucose oxidation pathway to glycolysis that occurs in the cytosol and generates reducing equivalents in the form of NADPH as well as pentoses for nucleic acid synthesis.
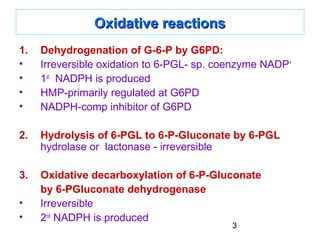
2. Glucose-6-phosphate enters the oxidative phase of the pathway where it is dehydrogenated by G6PD, producing NADPH. It is then hydrolyzed and decarboxylated further producing a second NADPH molecule.
3. The non-oxidative phase interconverts
![1
HMP SHUNT [Hexose Monophosphate Pathway] /HMP SHUNT [Hexose Monophosphate Pathway] /
PPP [Pentose Phosphate Pathway]PPP [Pentose Phosphate Pathway]
• Principle pathway for oxidation of Glucose-Glycolysis & TCA
• HMP-alternative pathway for oxidation of Glucose-not for Energy
• Occours in cytosol
• Provides: NADPH [reductive synthesis] & Pentoses [for NA synth]
• Most common at the site of synthesis:
Liver [phospholipid, FA synth, Cholesterol], adipose tissue [FA synth],
lactating mammary gland [FA synth], adrenal cortex [Cholesterol, steroid
hormone synth], testes & other endocrine glands concerned with steroid
synthesis and RBC
• NADPH produced: required for – Reductive biosynthesis of FA, TG,
cholesterol, steroids
[NADH – reduction in Catabolic pathways (NADH enters ETC→ ATP);
NADPH – reduction in Synthetic pathways]](https://image.slidesharecdn.com/hmpshunt-140712142729-phpapp02/85/Hmp-shunt-1-320.jpg)

![4
Non-oxidative reactionsNon-oxidative reactions
• Interconversion of 3-, 4-, 5- and 7-Carbon Sugars
• Ribulose 5-P to be converted to ribose 5-P [for Nucleic
acid synthesis]
• Or to intermediates of Glycolysis : F-6-P and
Glycerladehyde-3-P
• Non-oxidative part - controlled by availability of
intermediates
• Only coenzyme required: TPP for Transketolase reaction](https://image.slidesharecdn.com/hmpshunt-140712142729-phpapp02/85/Hmp-shunt-4-320.jpg)

![6
Uses of NADPHUses of NADPH
1.1. Reductive biosynthesis:Reductive biosynthesis:
NADPH is a high energy molecule and electron donor. It is
required as a source of electrons for biosynthesis of FA,
cholesterol, sterols, hormones, and bile salts.
2.2. Reduction of HReduction of H22OO22:: ROS- damage DNA, proteins, unsaturated
lipids– reperfusion injuries, cancer, inflammatory diseases,
aging.
Several protective mechanisms:
a. Enzymes that catalyze Antioxidant reactions:
Glutathione peroxidase or GOD [ using reduced Glutathione or
GSH which is active as antioxidant, present in most cells which
can detoxify HH22OO22 ]
Glutathione reductase or GR [regenerates GSH from oxidized
glutathione formed in above reaction] using NADPHNADPH as a source
of electrons
b. Antioxidant chemicals:
Vit E, A, C, uric acid, bilirubin, ceruloplasmin etc](https://image.slidesharecdn.com/hmpshunt-140712142729-phpapp02/85/Hmp-shunt-6-320.jpg)
![7
3.3. CYP 450 or Cyt P-450: major pathway for hydroxylation ofCYP 450 or Cyt P-450: major pathway for hydroxylation of
xenobioticsxenobiotics
Supply of NADPHNADPH is critical for liver microsomal cyt P-450
4.4. Phagocytosis of microorganisms esp bacteria, foreignPhagocytosis of microorganisms esp bacteria, foreign
particles etc by Neutrophils & Macrophages:particles etc by Neutrophils & Macrophages:
imp defense mechanism
a. Oxygen dependent system- MPO [myeloperoxidase system]-
most potent, NADPH OXIDASE [needs NADPH] in WBC cell
memb, converts OO22 into Superoxide FRinto Superoxide FR [Respiratory
burst]→Superoxide is converted by SOD [superoxid dismutase]
into HH22OO22 →lysosomal MPO converts it to hypochlorous acid
HOCl·→ kills bacteria
NADPH OXIDASE deficiency- Chronic granulomatosis
b. Oxygen independent system- pH changes in phagolysosomes
and lysosomal enzymes- destroy pathogens](https://image.slidesharecdn.com/hmpshunt-140712142729-phpapp02/85/Hmp-shunt-7-320.jpg)

![9
G-6-PD deficiency
• Inherited disease-most common disease producing enzyme
abnormality in humans- X-linked
• Hemolytic anemia due to inability to detoxify oxidizing agent
• 400 different types of mutations [point mutations]
• Shortened life span due to complications
• Increased resistance to falciparum malaria in female carriers of
mutation
• ↓activity of G6-PD→↓NADPH [HMP] →↓detoxification of FR &
peroxides
• RBC- most vulnerable as HMP is only means for NADPH
production. (other tissues NADP-dependent malate dehydrogenase
also]
• Precipitating factors- Oxidant drugs[ A-antibioticsA-antibiotics
(sulfamethoxazole), A-antimalarial (primaquin),A-antipyritics(sulfamethoxazole), A-antimalarial (primaquin),A-antipyritics
(acetanilid)(acetanilid), ingest Fava beans [favism], severe infection-free
radical generation in macrophages diffuse to RBC-hemolysis](https://image.slidesharecdn.com/hmpshunt-140712142729-phpapp02/85/Hmp-shunt-9-320.jpg)
![10
Regulatory Mechanisms
Enzymes G6PD and 6PGD catalyze irreversible
steps of the HMP shunt.
↑ [NADPH] inhibits these enzymes via feedback
inhibition
↑ [ATP]: a putative inhibitor of these steps
↑ [G-6P] increases flux through the HMP shunt (G-6P is a
substrate)](https://image.slidesharecdn.com/hmpshunt-140712142729-phpapp02/85/Hmp-shunt-10-320.jpg)

![12
Uronic acid Pathway of GlucoseUronic acid Pathway of Glucose
Importance in humans:
• Provides UDP-glucuronic acid for conjugation [conjugation of
bilirubin, steroids etc] and synthesis of glycosaminoglycans.
• In lower animals (not in primates- deficiency of enzyme L-
gulonolactone oxidase), this pathway leads to synthesis of Vit C.
• Essential Pentosuria: one of Garrod’s tetrad [alkaptonuria,
albinism, pentosuria, cystinuria- inborn error of metabolism]:
*1 in 2500 births due to deficiency of xylitol dehydrogenase →
L-xylulose excreted in urine gives + benedict’s test-not harmful.
*Diffentiated from DM by + Bials test [orcinol in HCL-Bial’s reagent]
by pentose sugars.](https://image.slidesharecdn.com/hmpshunt-140712142729-phpapp02/85/Hmp-shunt-12-320.jpg)
![13
URONIC ACID PATHWAYURONIC ACID PATHWAY
G-6-PG-6-P
PhosphoglucomutasePhosphoglucomutase
G-1-PG-1-P
+ UTP [UDPG Phosphorylase]UTP [UDPG Phosphorylase]
UDP- GlucoseUDP- Glucose
enters Uronic acid pathway](https://image.slidesharecdn.com/hmpshunt-140712142729-phpapp02/85/Hmp-shunt-13-320.jpg)
![14
Aldose reductaseAldose reductase- Glucose to Sorbitol [glucitol]:
Lens, retina, Schwann cell of peripheral nerves, kidney, placenta,
RBC, cells of ovaries and seminal vesicles.
Sorbitol dehydrogenaseSorbitol dehydrogenase- Sorbitol to Fructose
Glucoe to Sorbitol to Fructose: in seminal vesicles for sperm cell [fructose is
preferred carbohydrate energy source]
Hyperglycemia: Uncontrolled DM-large amt Glucose entersHyperglycemia: Uncontrolled DM-large amt Glucose enters
Lens, Retina,Nerve, Kidney – with action of aldose reductaseLens, Retina,Nerve, Kidney – with action of aldose reductase
→↑→↑Sorbitol, cannot pass through cell memb, so trapped insideSorbitol, cannot pass through cell memb, so trapped inside
cell.cell.
Sorbitol dehydrogenase is absent in Lens, retina, kidney andSorbitol dehydrogenase is absent in Lens, retina, kidney and
nerve cell →↑sorbitol accumulates →Osmotic effects →cellnerve cell →↑sorbitol accumulates →Osmotic effects →cell
swelling and water retention:swelling and water retention:
cause of cataract formation, peripheral neuropathy, vascularcause of cataract formation, peripheral neuropathy, vascular
problems leading to nephropathy and retinopathyproblems leading to nephropathy and retinopathy](https://image.slidesharecdn.com/hmpshunt-140712142729-phpapp02/85/Hmp-shunt-14-320.jpg)