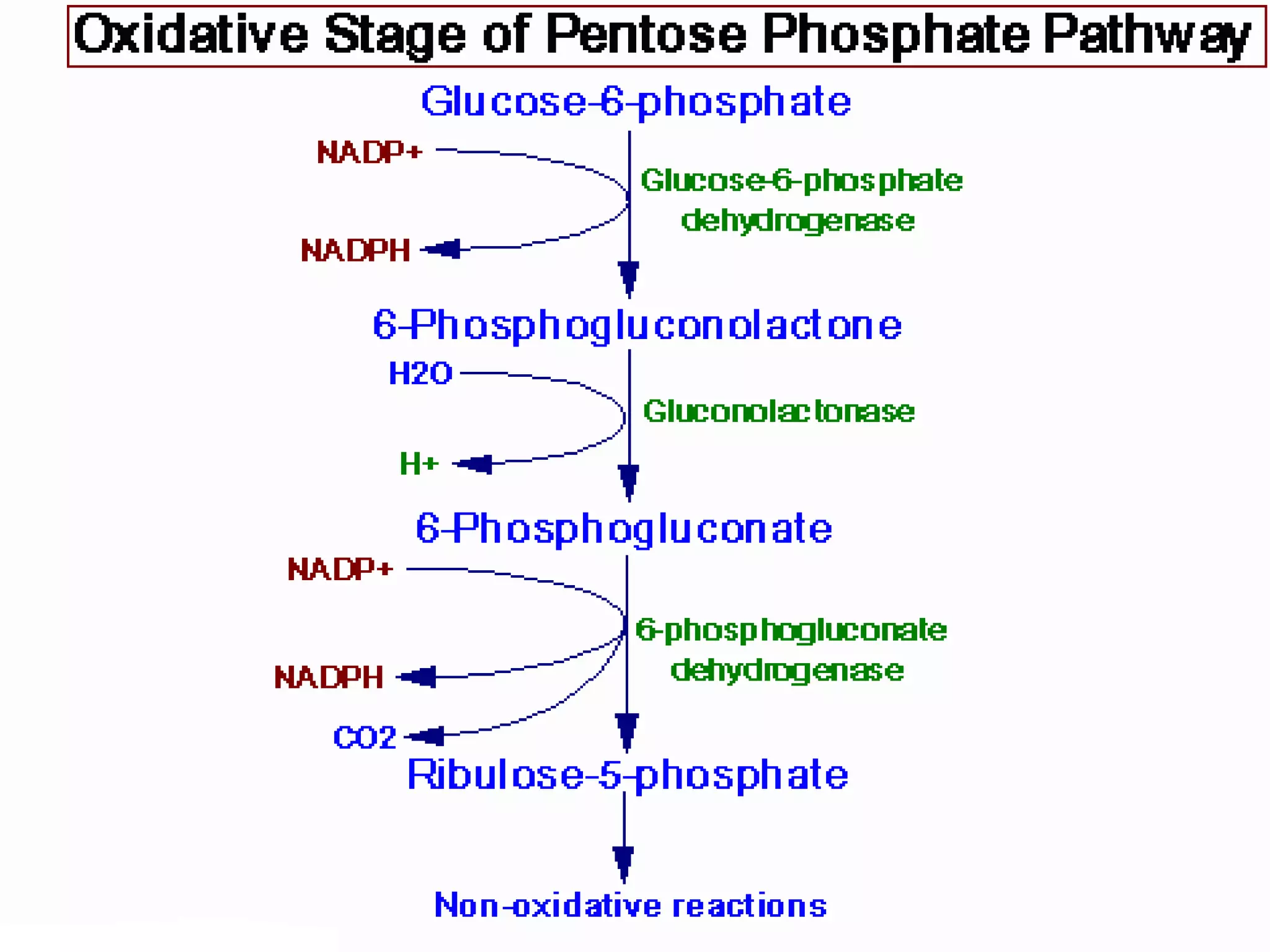
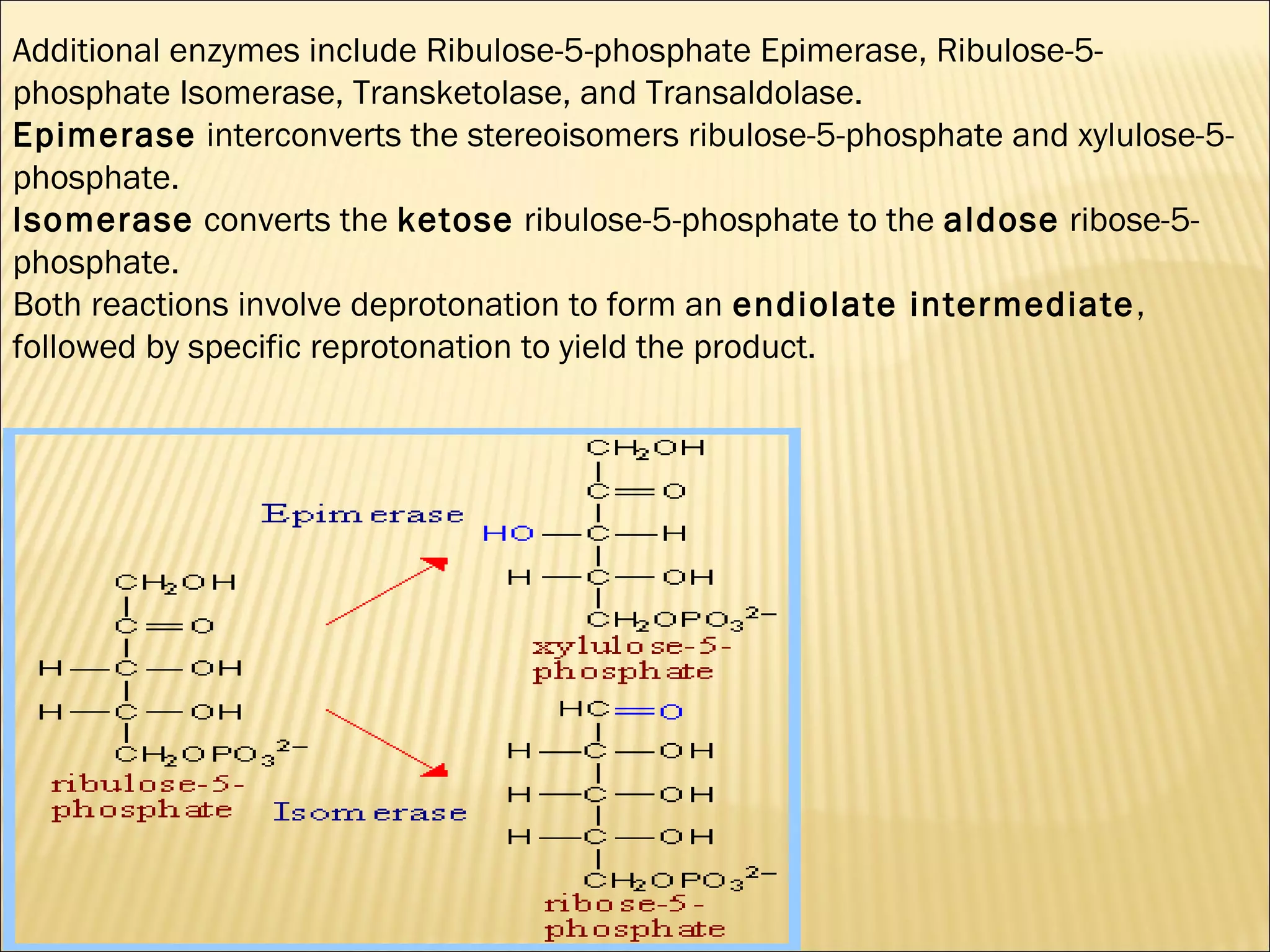
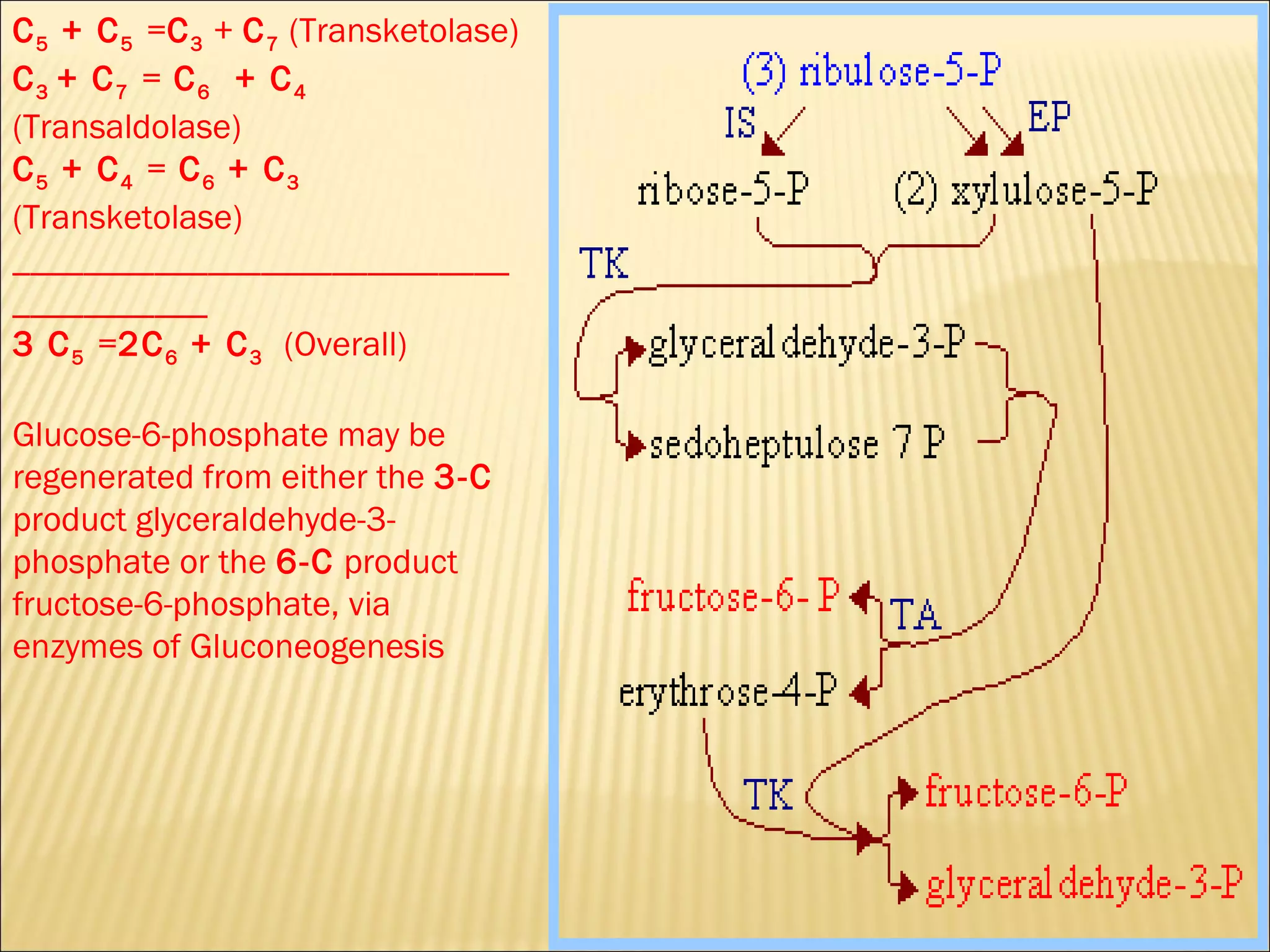
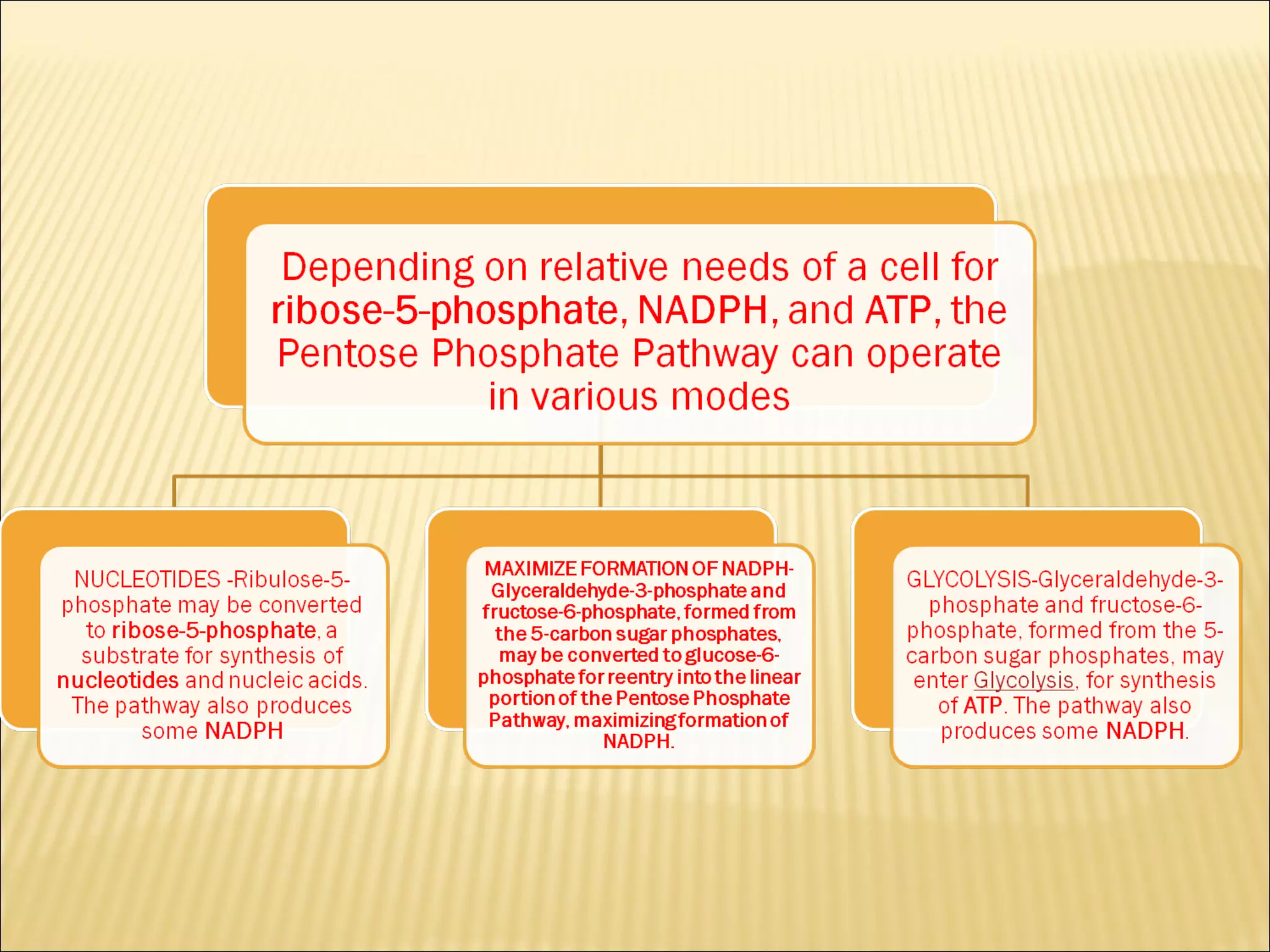
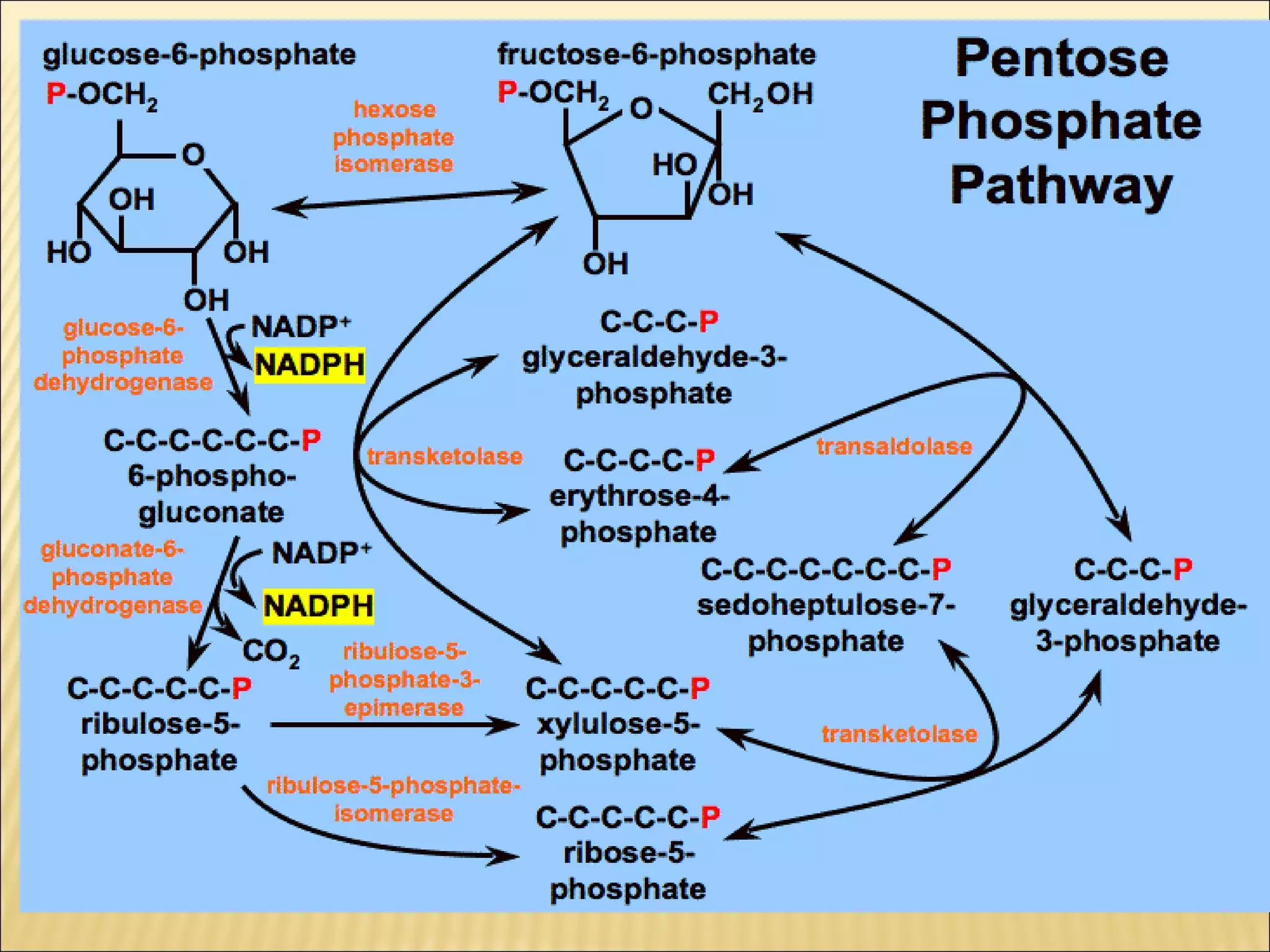
The pentose phosphate pathway generates reducing equivalents in the form of NADPH and produces ribose-5-phosphate. It functions primarily in anabolic biosynthesis reactions that require NADPH, such as fatty acid and nucleic acid synthesis. Cells with high levels of these biosynthetic pathways, such as liver, adipose, and lactating mammary gland cells, have high levels of pentose phosphate pathway enzymes. The pathway oxidizes glucose through a series of reactions that ultimately produce ribulose-5-phosphate, which can be converted back into glucose-6-phosphate or other three and six carbon intermediates.