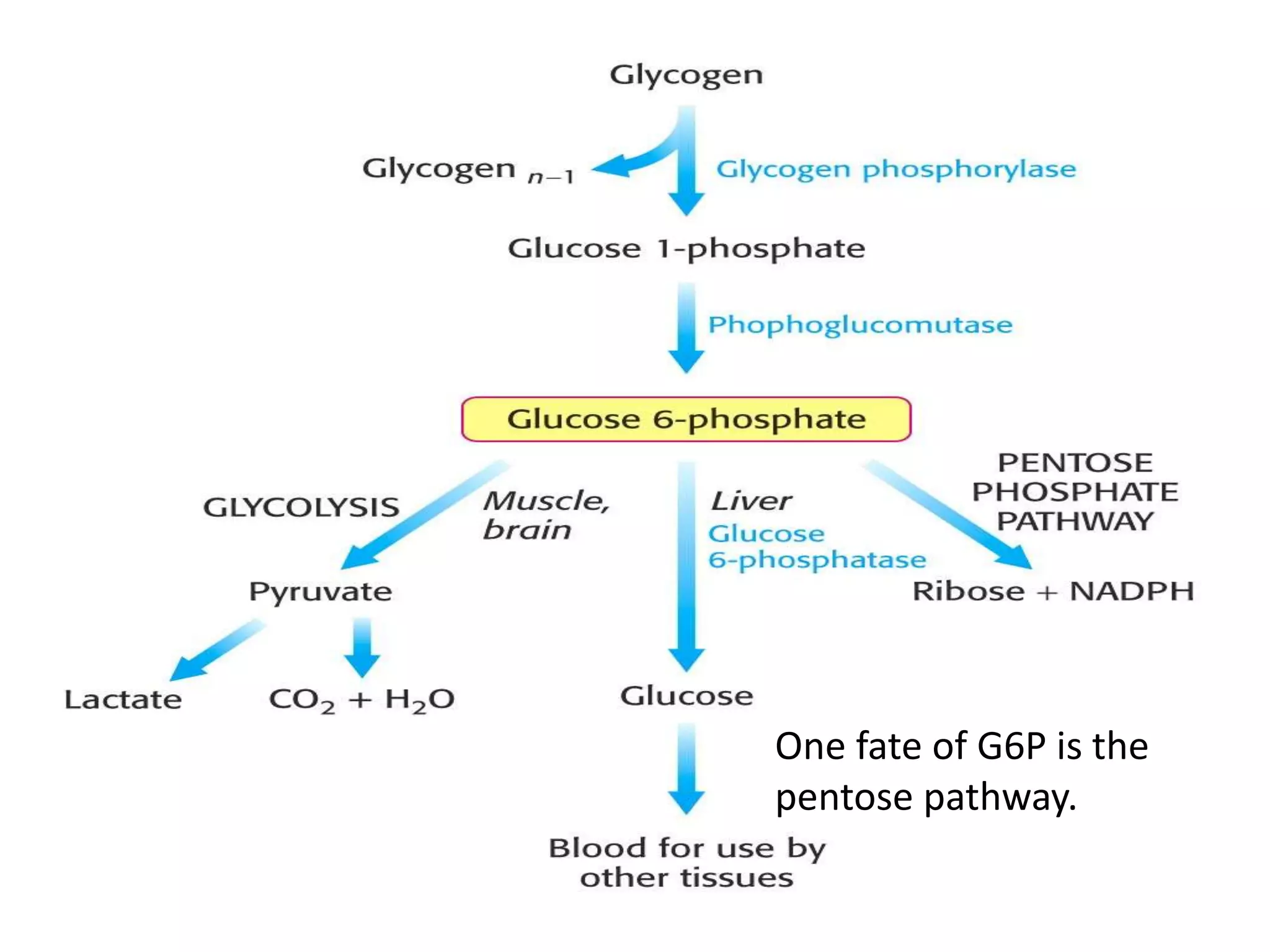
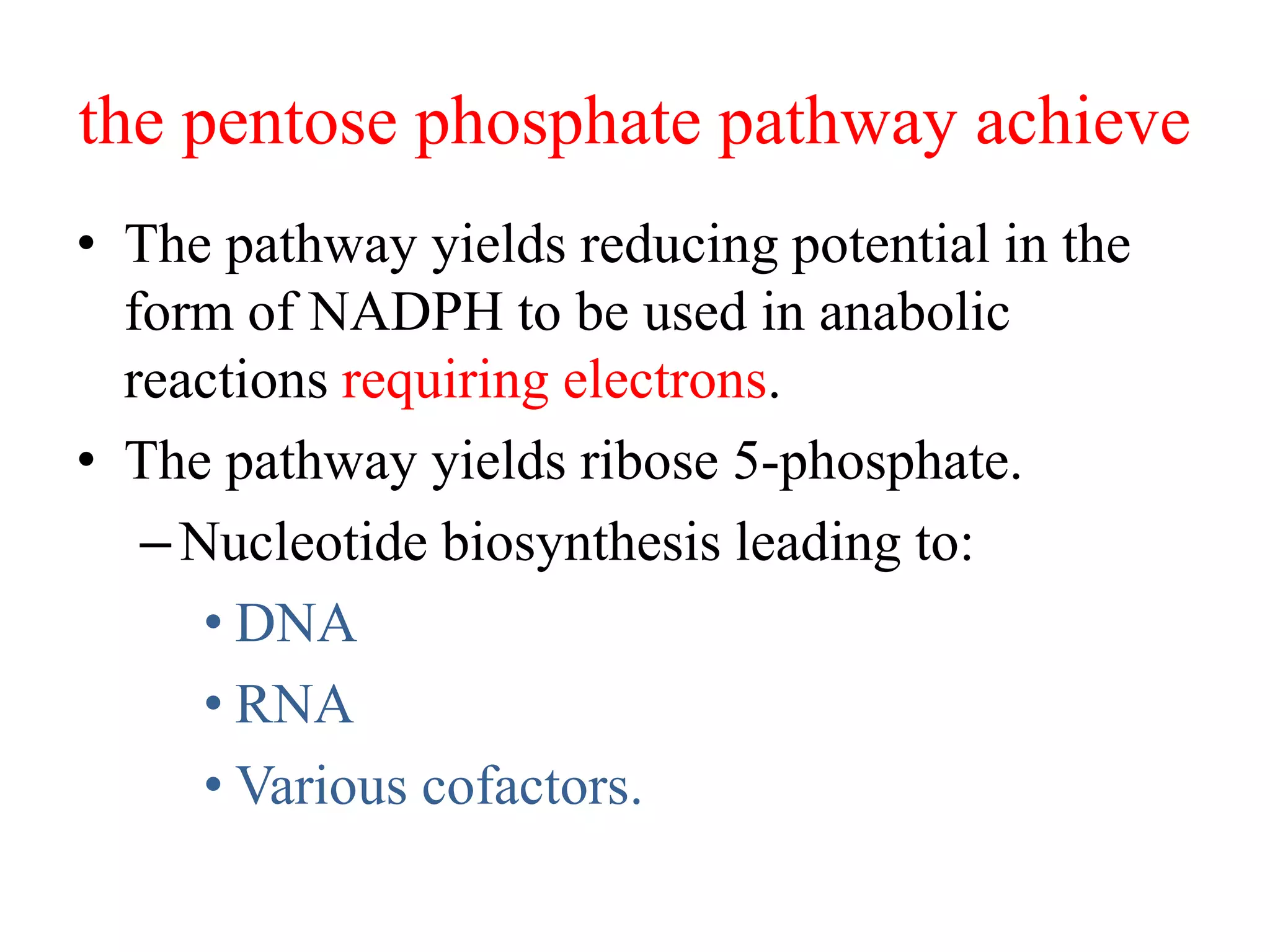
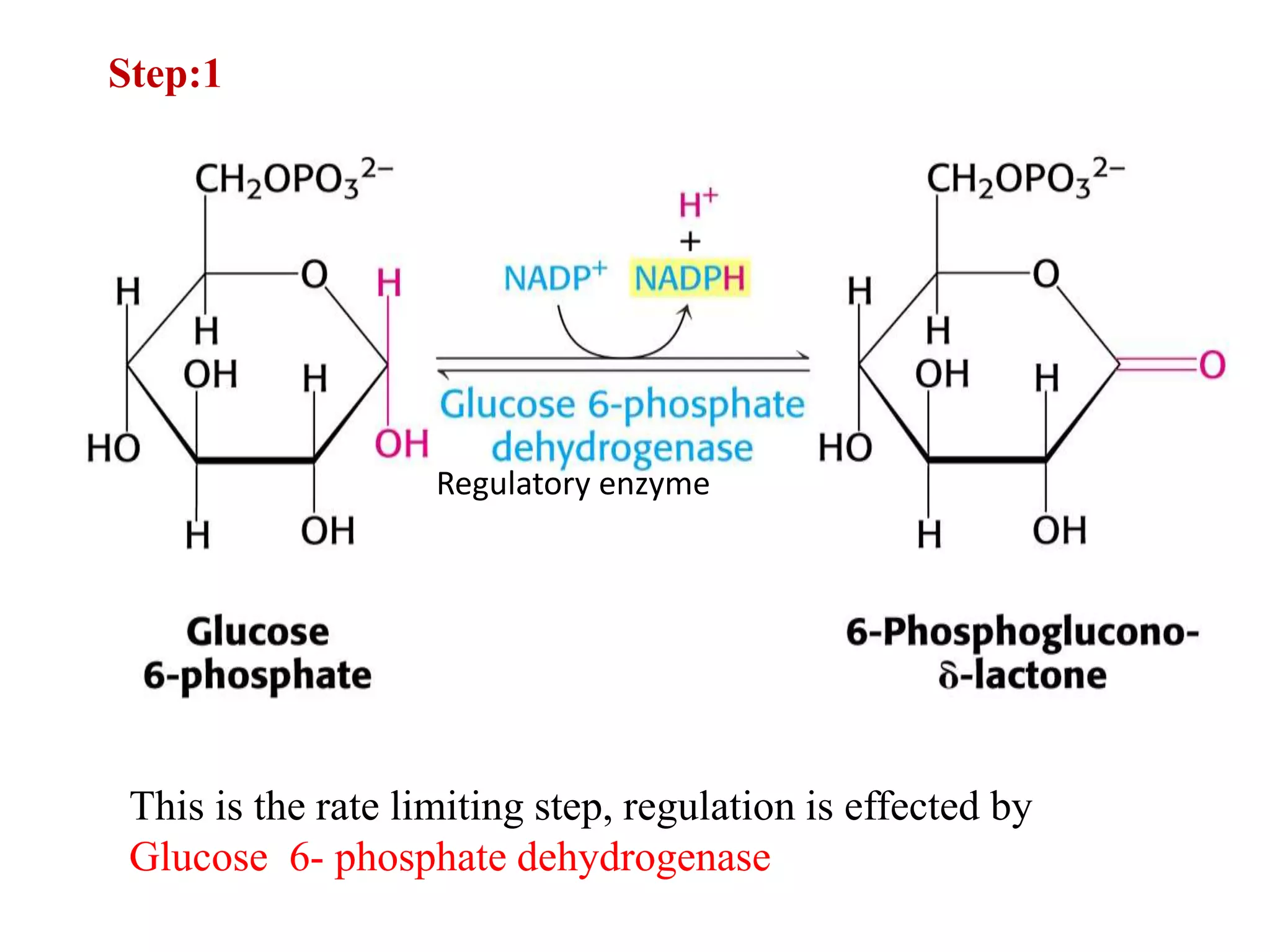
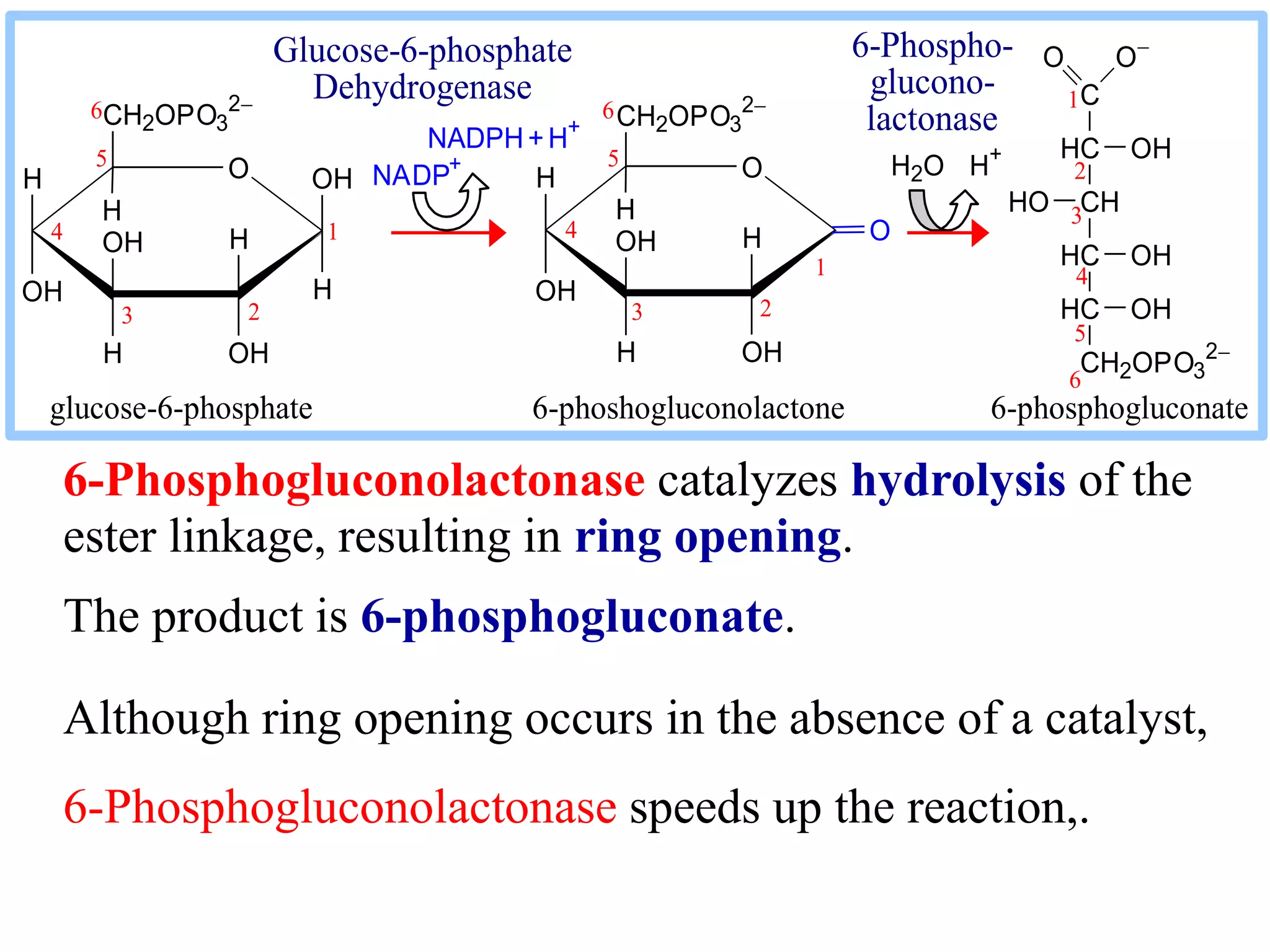
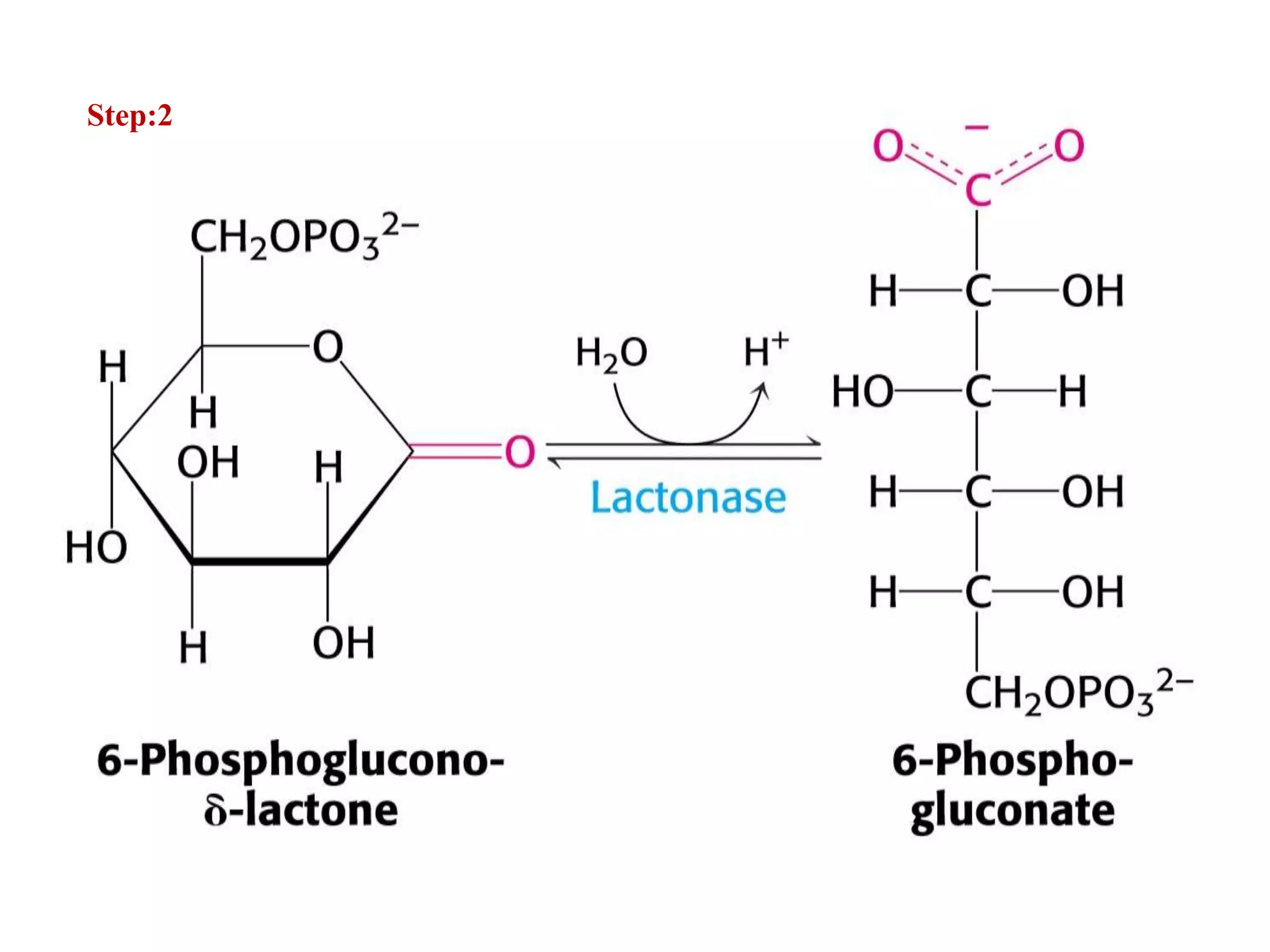
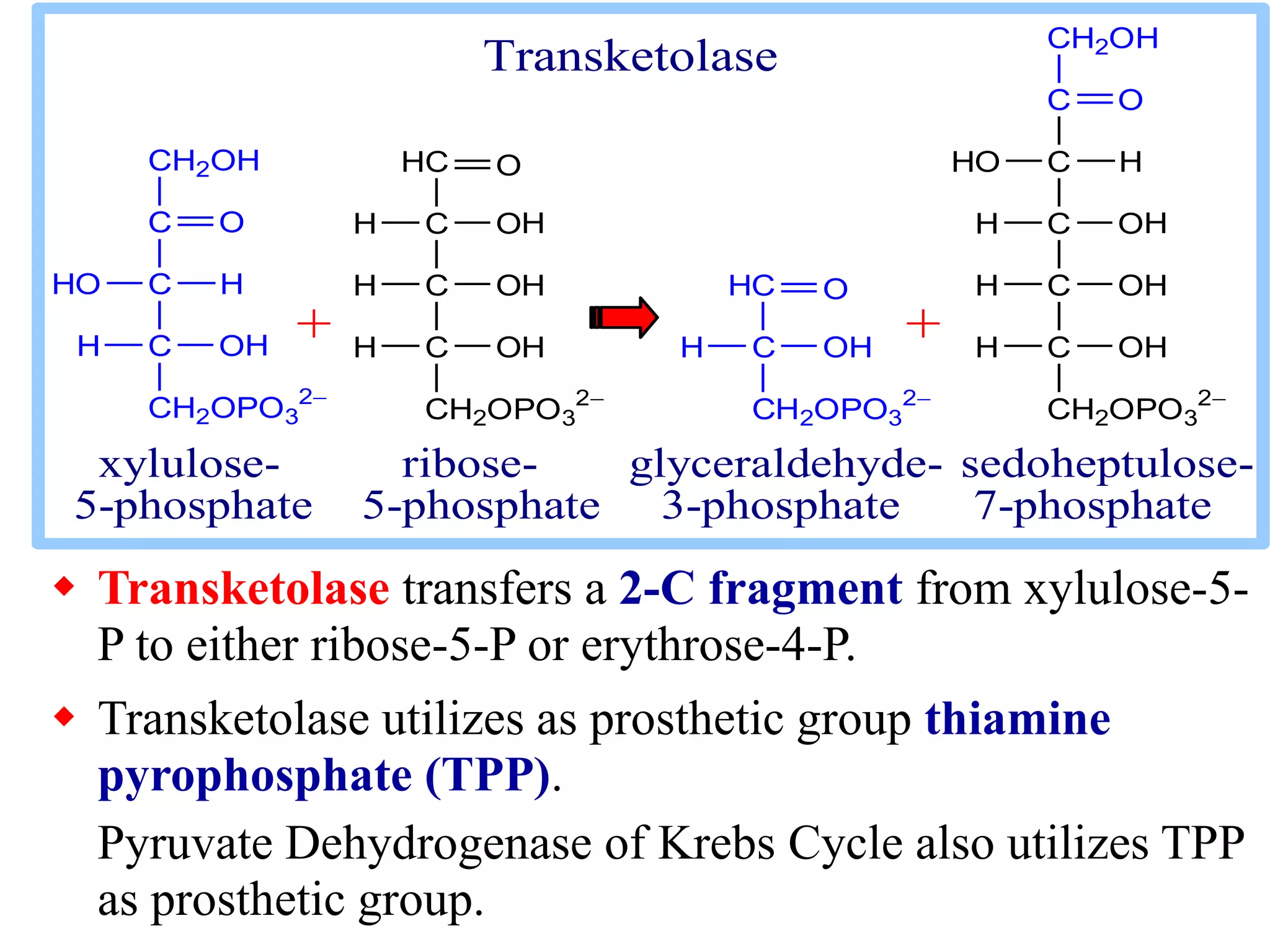
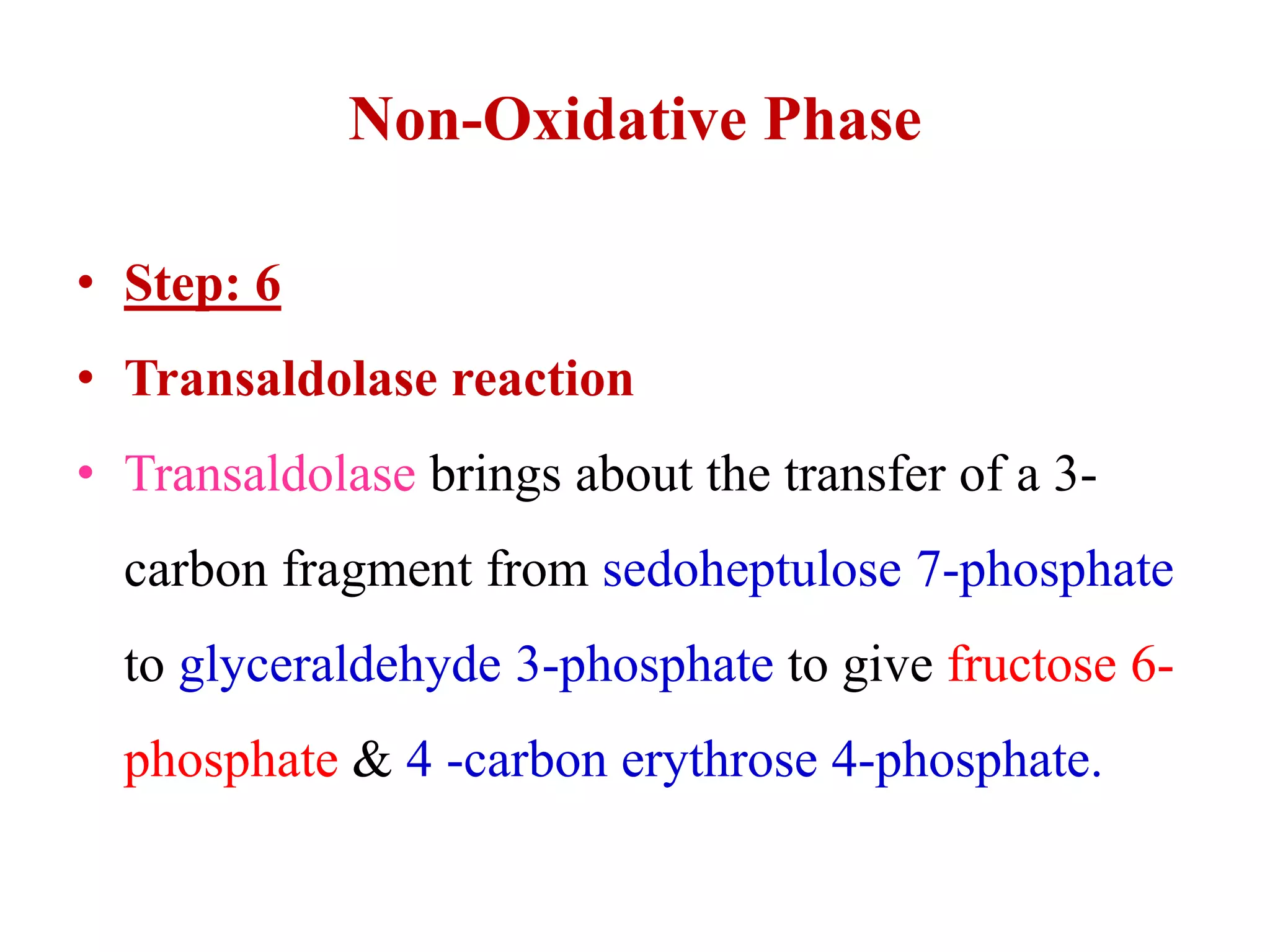
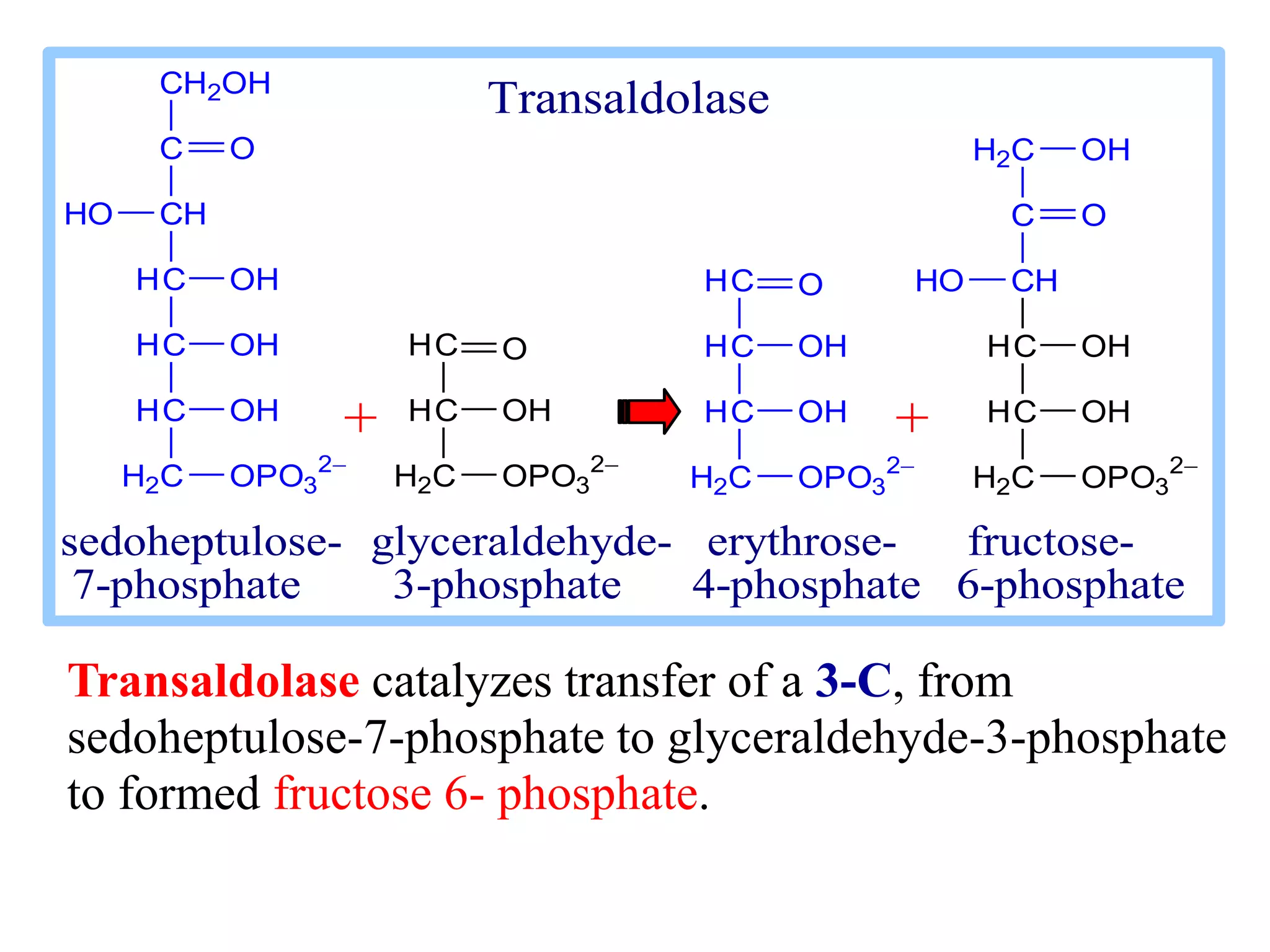
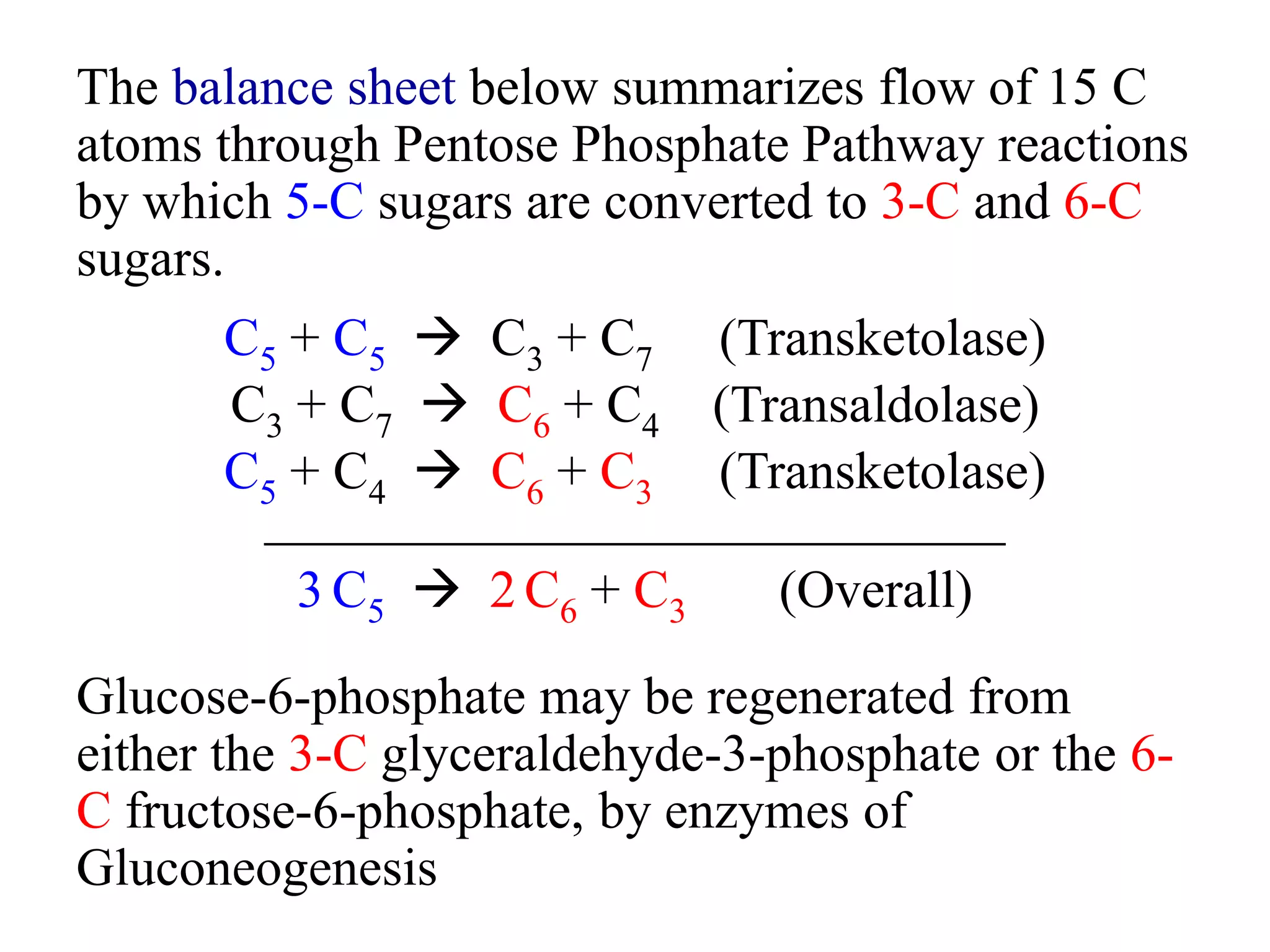
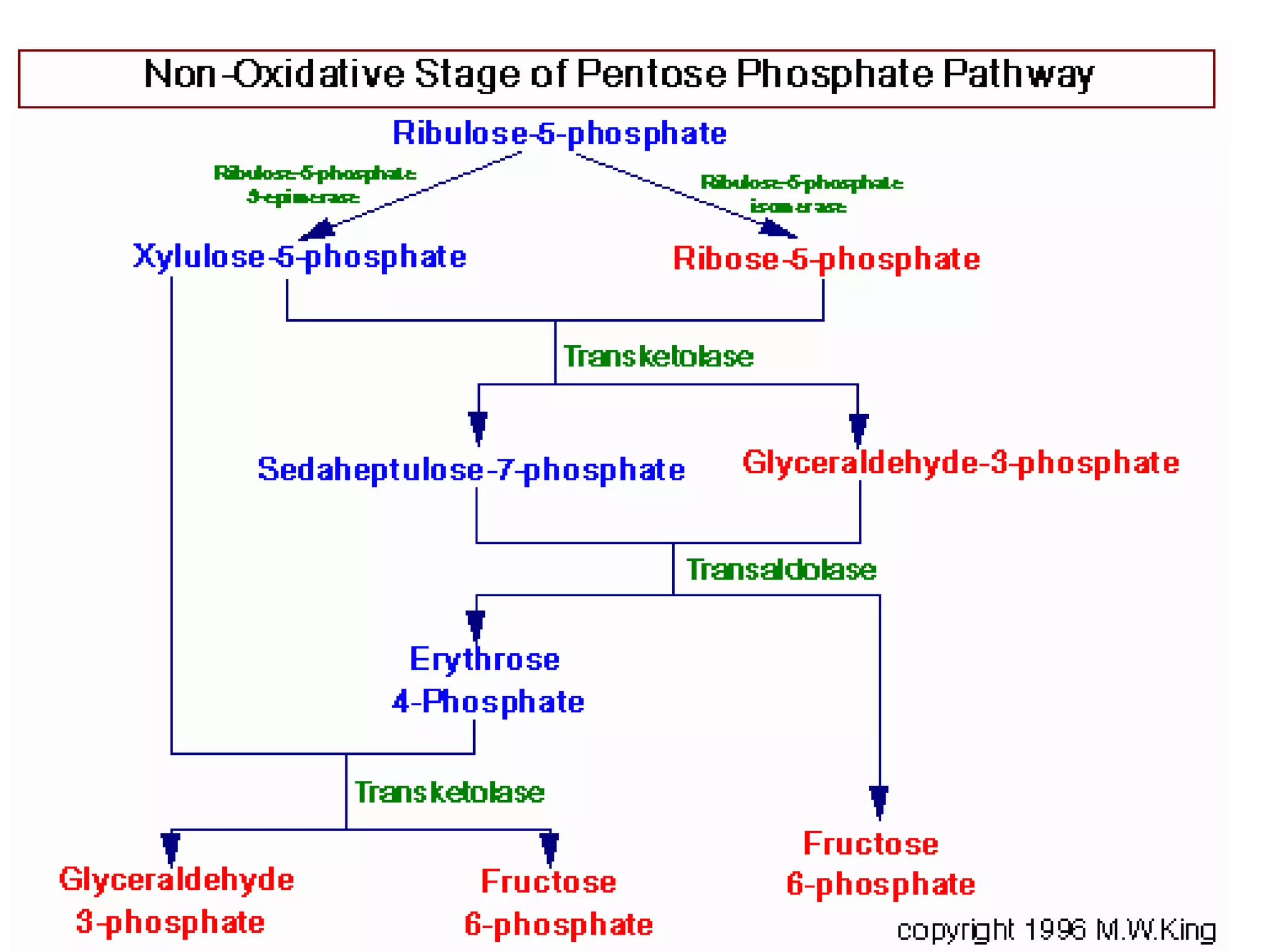
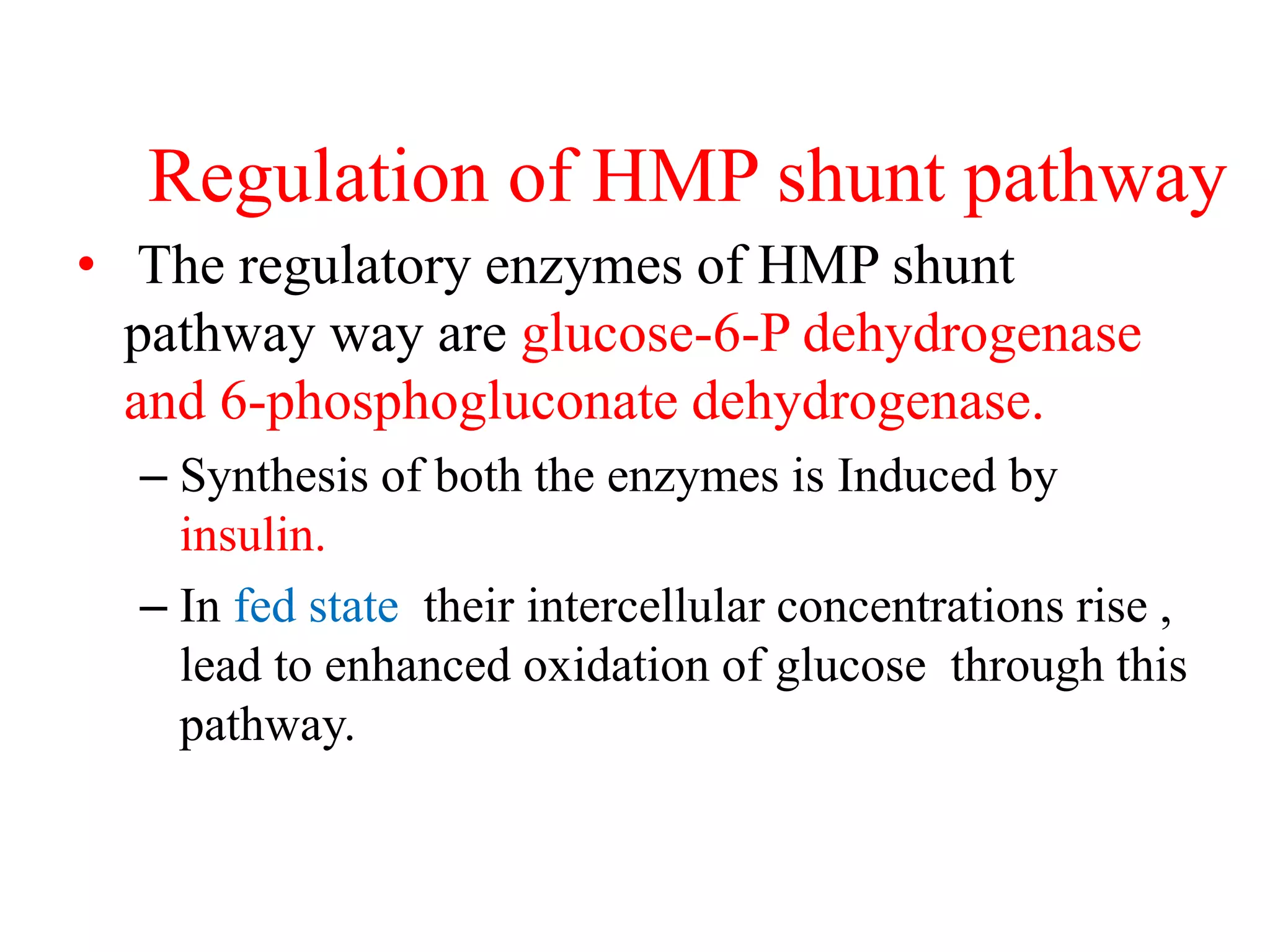
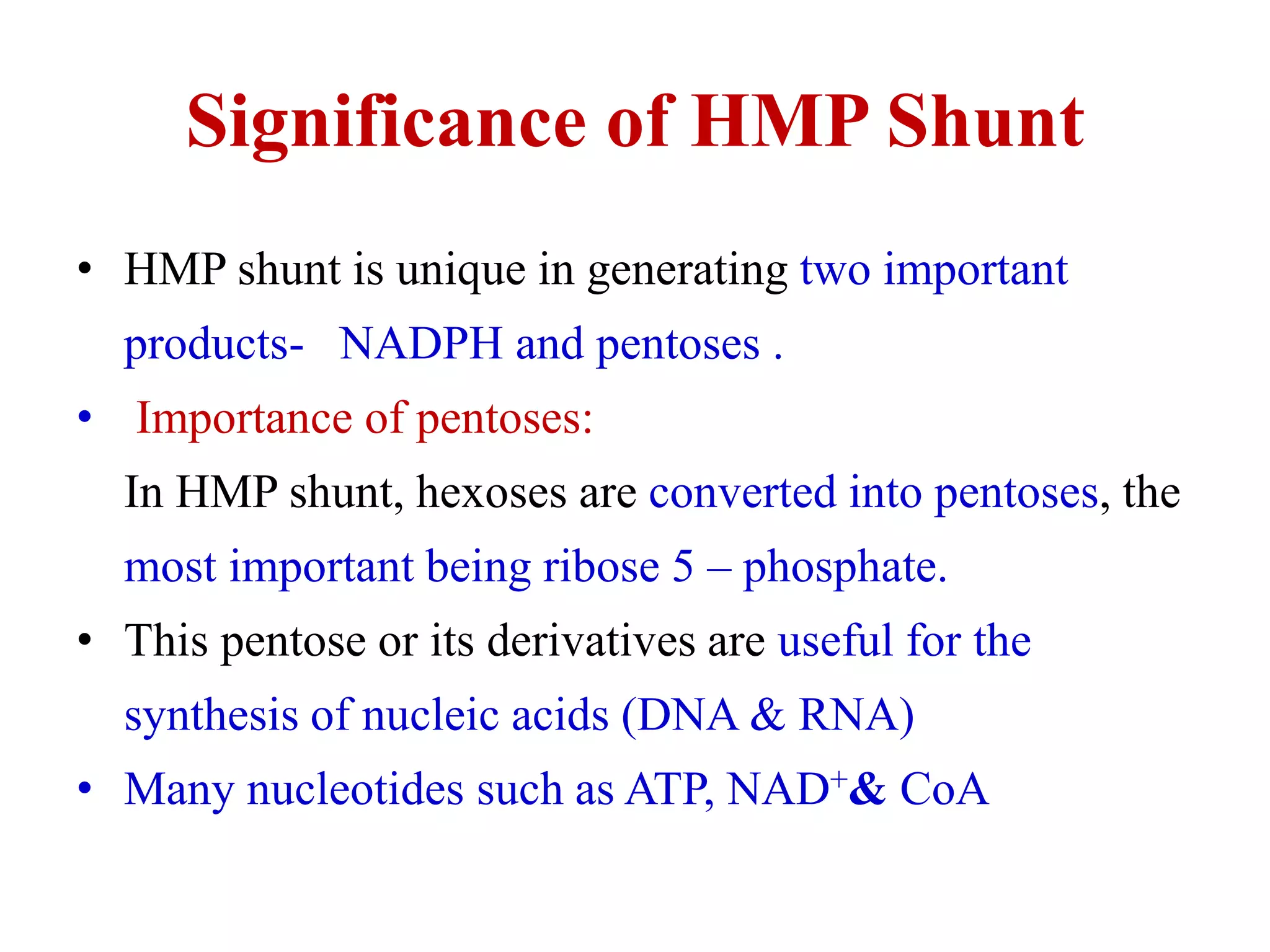
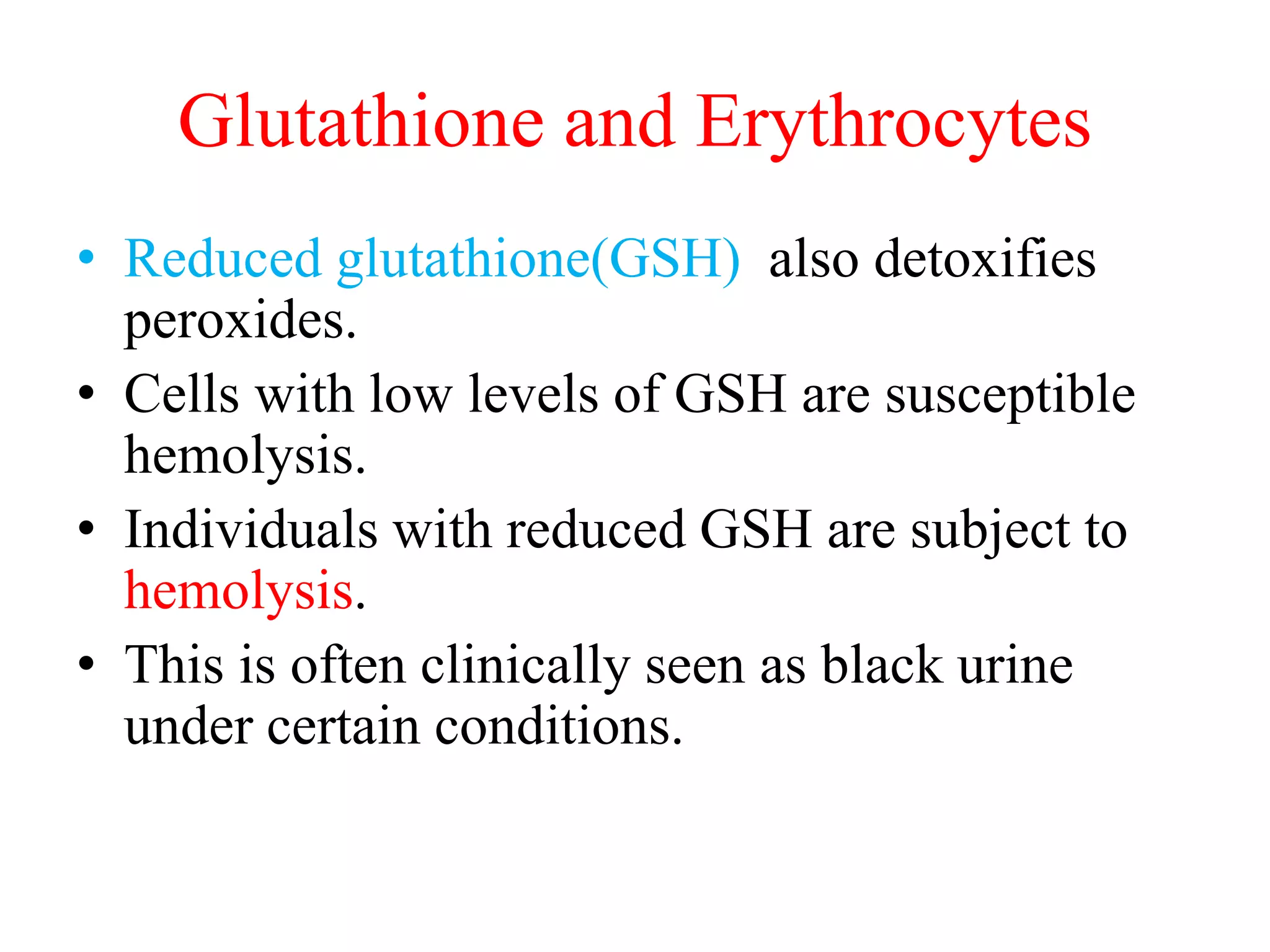
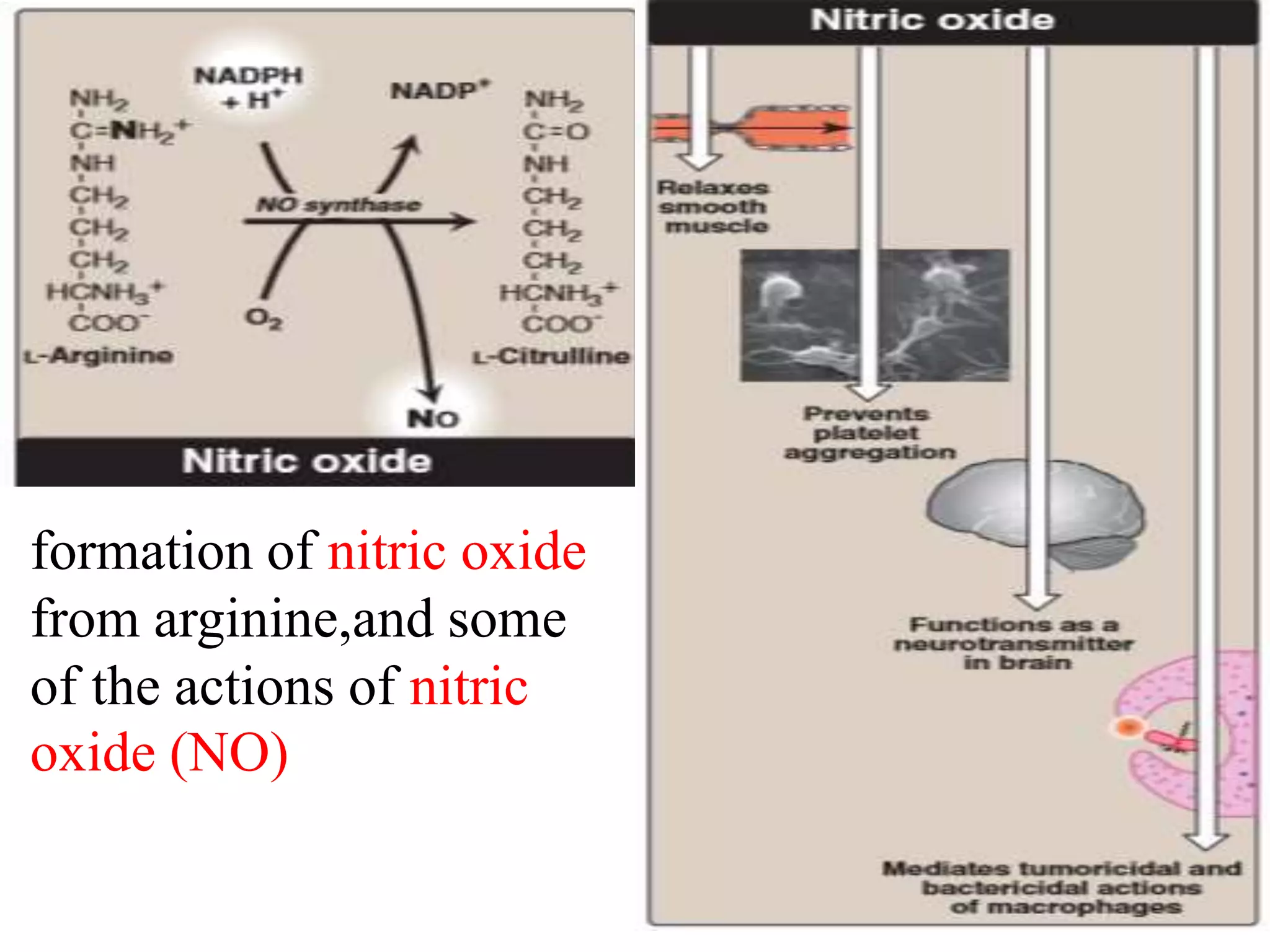
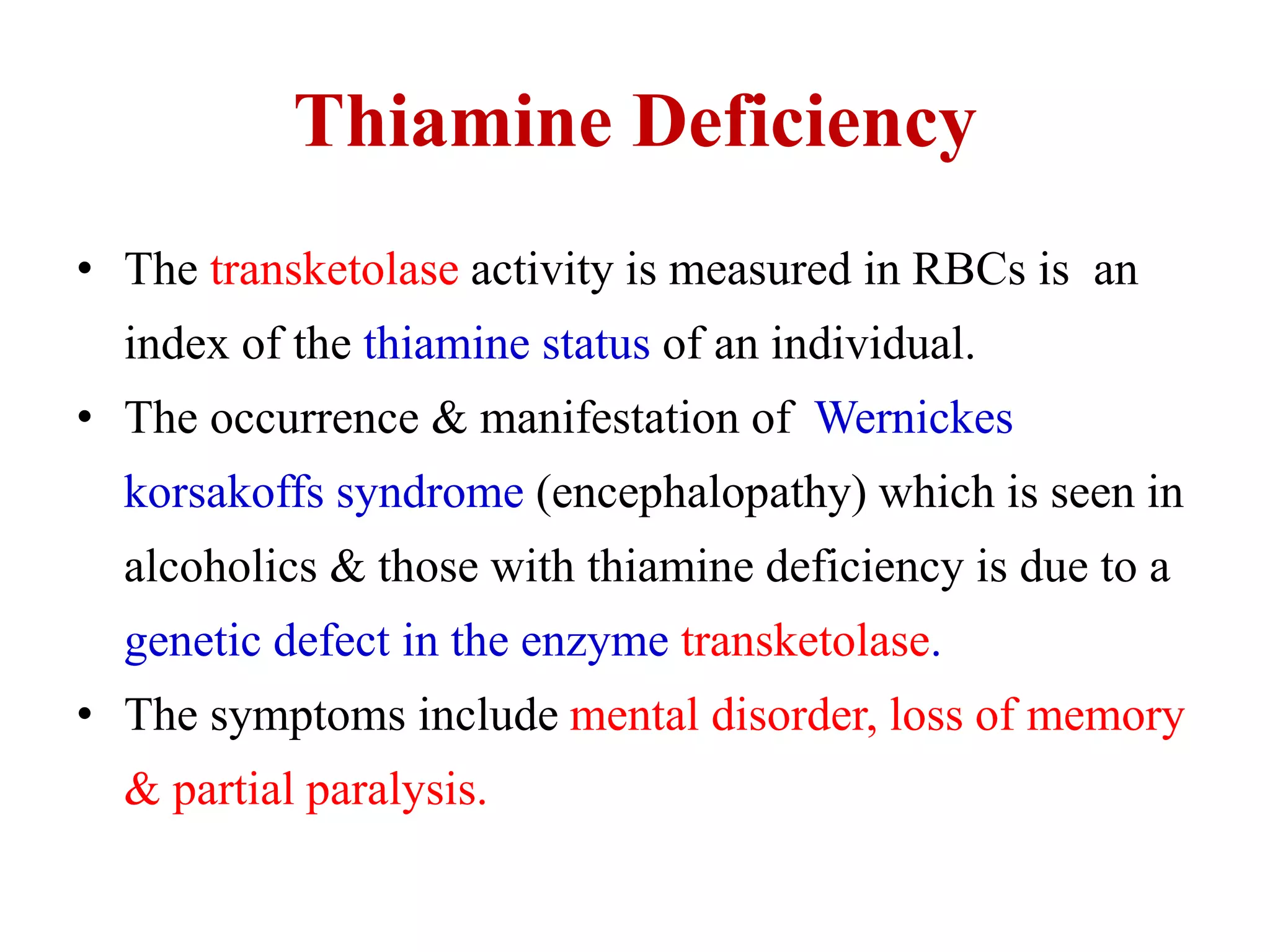
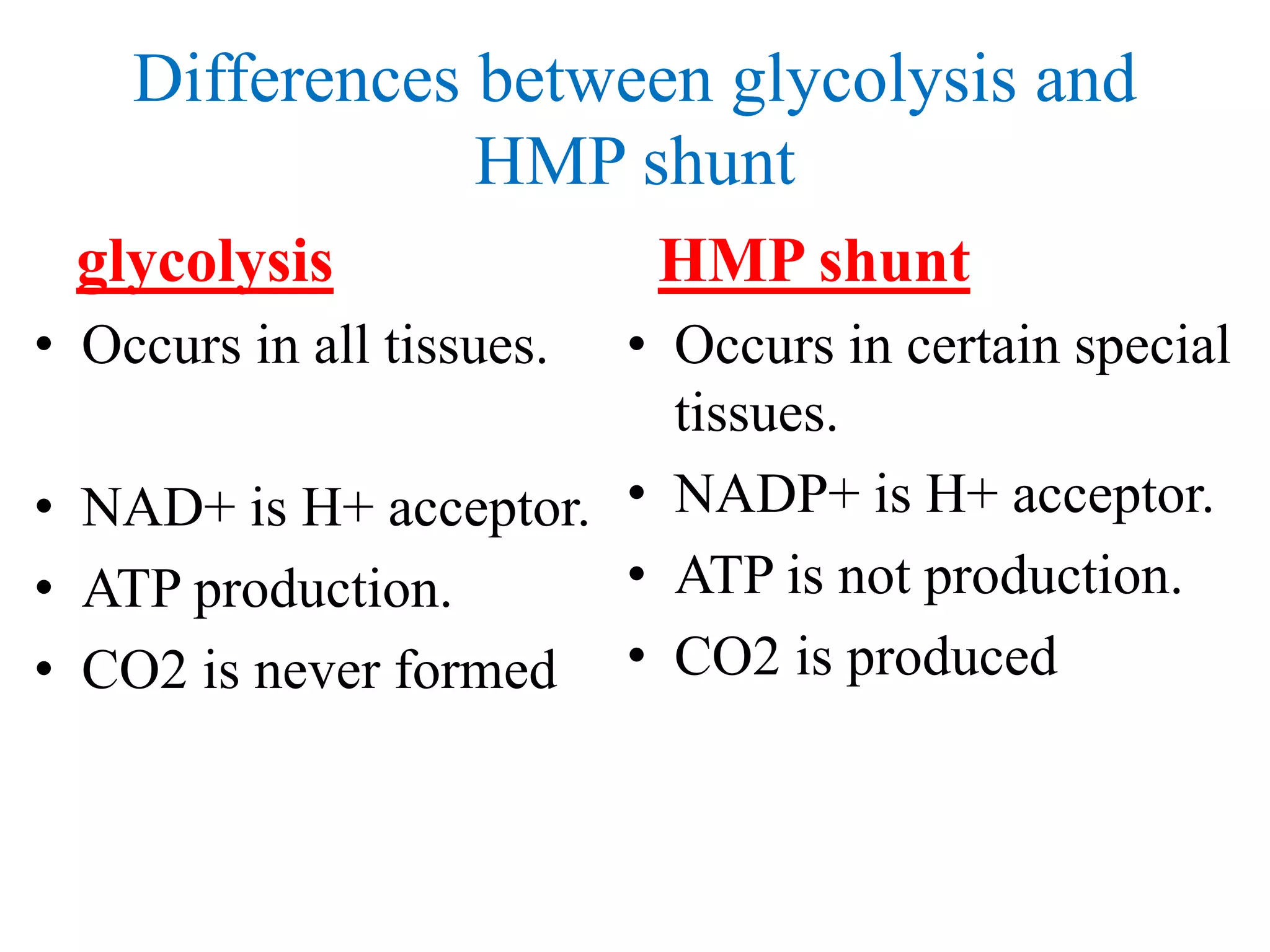
The pentose phosphate pathway generates NADPH and pentose sugars. It has both an oxidative and non-oxidative phase. In the oxidative phase, glucose-6-phosphate is oxidized to produce NADPH. In the non-oxidative phase, 5-carbon sugars are converted into 3 and 6 carbon sugars through a series of isomerization, epimerization, and transketolase reactions. The pathway is important as it provides NADPH for biosynthetic reactions and pentose sugars for nucleotide synthesis. A defect in glucose-6-phosphate dehydrogenase can lead to insufficient NADPH and glutathione, resulting in hemolytic anemia due to oxidative damage of red blood cells.