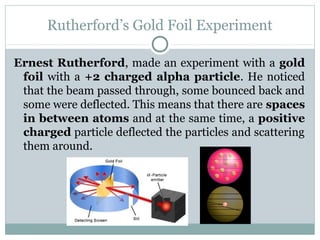
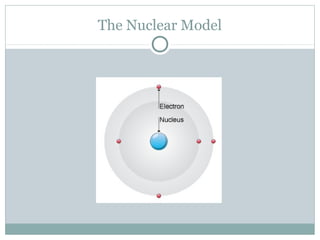
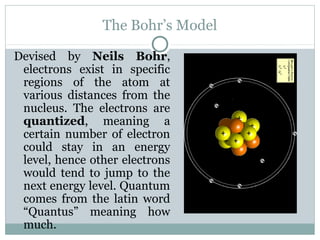
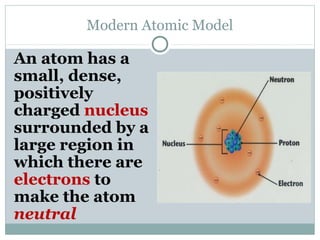
The document discusses the history of atomic theory from ancient Greek philosophers' ideas of "primal matter" to modern atomic structure. It describes early atomic models including Thomson's "plum pudding" model and Rutherford's gold foil experiment. Key developments included Dalton's atomic theory, the discovery of subatomic particles like the electron, proton, and neutron, and the modern nuclear model with electrons orbiting a small, dense, positively-charged nucleus.