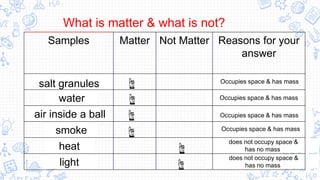
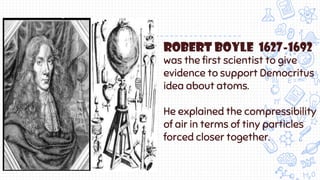
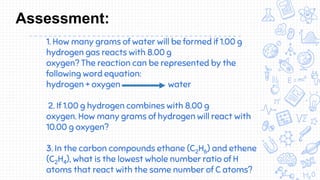
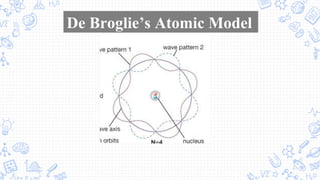
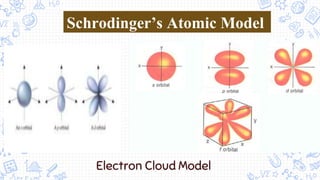
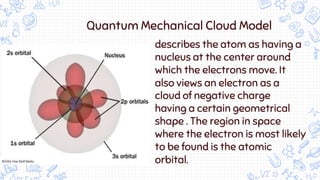
1. The document outlines the development of atomic theory from ancient Greek philosophers like Democritus and Aristotle to modern scientists like Dalton, Thomson, Rutherford, Bohr, Schrodinger, Heisenberg, and Chadwick.
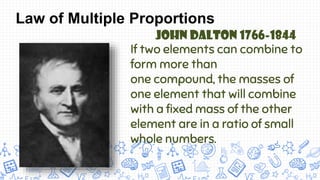
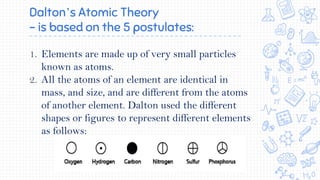
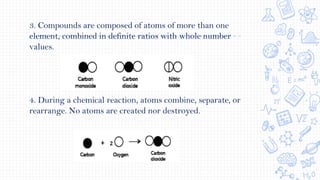
2. Key early atomic theories included Democritus' idea that matter is made of indivisible atoms and Dalton's atomic theory that elements are made of atoms that are unique in mass and size and that compounds are formed by combinations of atoms.
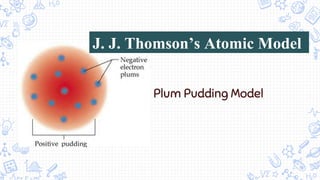
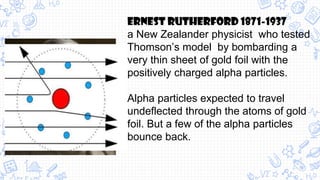
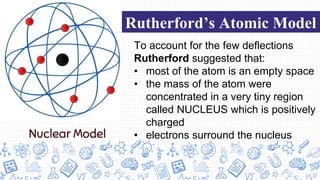
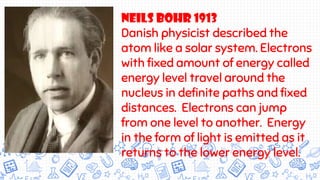
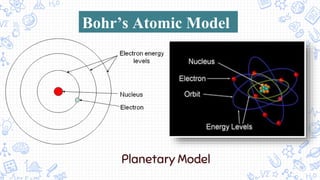
3. Modern atomic theories incorporated subatomic particles like the electron discovered by Thomson, the nuclear model developed by Rutherford after discovering alpha particle deflections, and Bohr's planetary model explaining electron energy levels.