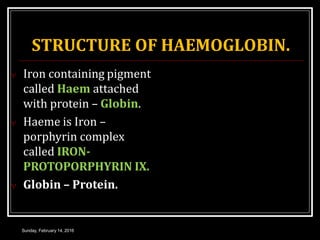
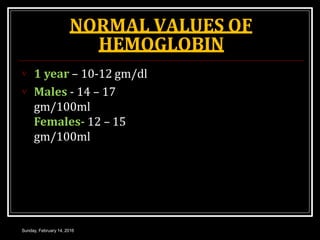
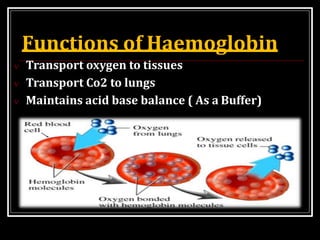
Hemoglobin is the red pigment found in red blood cells that carries oxygen from the lungs to tissues and carbon dioxide from tissues back to the lungs. It is a conjugated protein made up of an iron-containing heme group and a globin protein. Each hemoglobin molecule contains four heme groups with iron atoms that can each bind one oxygen molecule. There are different types of hemoglobin including adult hemoglobin A, fetal hemoglobin, and pathological hemoglobins involved in conditions like sickle cell disease and thalassemia. The structure and function of hemoglobin allows it to efficiently transport oxygen and carbon dioxide in the bloodstream.