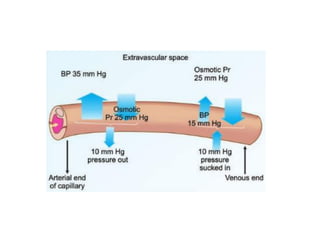
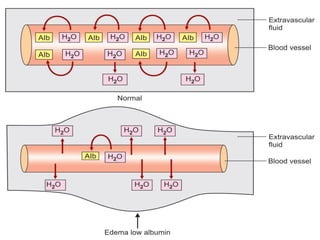
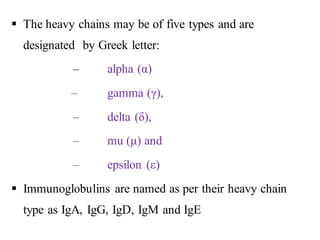
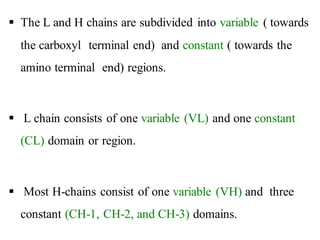
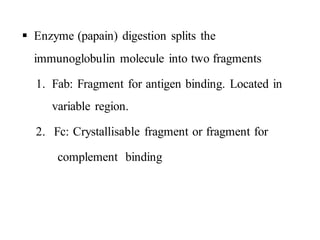
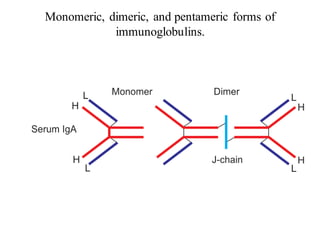
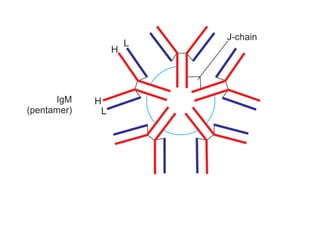
The document summarizes plasma proteins and immunoglobulins. It states that total plasma proteins are 6-8 g/dl, consisting mainly of albumin, globulins, and fibrinogen. Albumin and fibrinogen are synthesized solely by the liver, while globulins are produced in both the liver and lymphoid tissues. Immunoglobulins constitute approximately 20% of plasma proteins and are produced by plasma cells and lymphocytes in response to antigens. The five main classes of immunoglobulins are IgG, IgA, IgM, IgD, and IgE, which have different structures and functions.