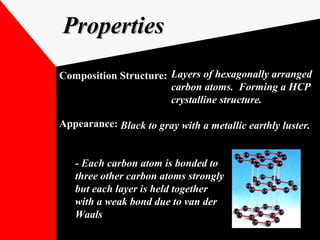
Graphite is a naturally occurring mineral made of layers of carbon atoms arranged in a hexagonal structure. It was first discovered in 1565 in Britain but can also be found in other parts of the world. Graphite is an excellent conductor of electricity with high strength and thermal stability. It has various applications including use as a moderator in nuclear reactors, as a lubricant in manufacturing, and when mixed with clay to form the "lead" in pencils. Graphite is also used to produce dry lubricants and paints.