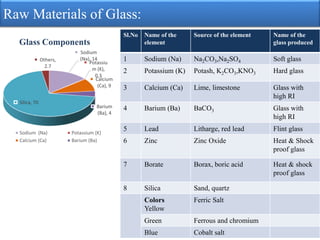
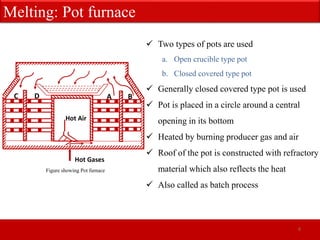
The document provides an extensive overview of glass, detailing its properties, raw materials, manufacturing processes, and various types of glass. It describes the melting process in pot and tank furnaces as well as the subsequent forming, shaping, annealing, and finishing steps, leading to the production of different types of glass, such as soda-lime, lead, and borosilicate glass. Each type has unique compositions, properties, and uses, with specific raw material requirements.