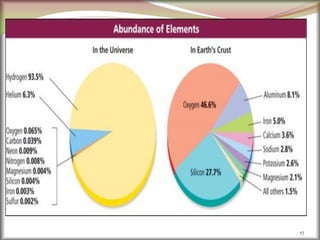
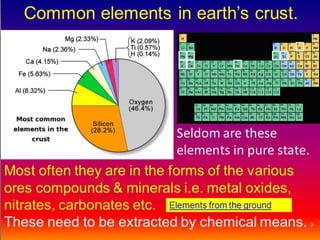
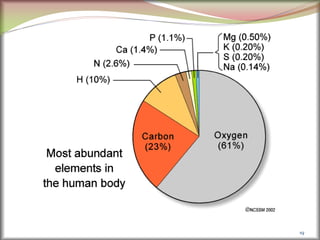
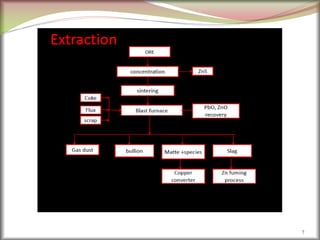
The document discusses how metals occur in nature and the processes involved in extracting metals from ores. It makes the following key points:
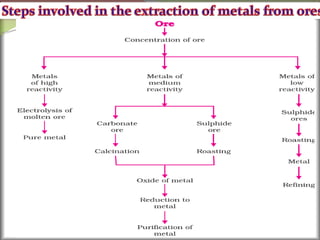
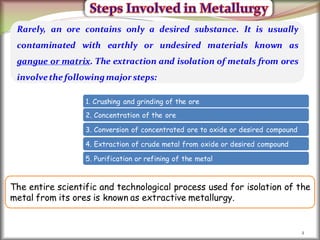
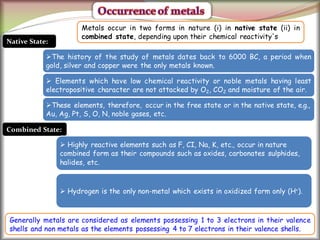
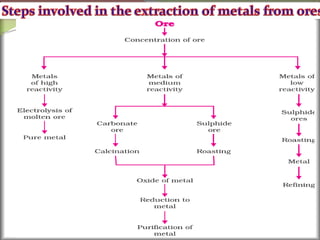
1. Metals occur naturally in either a native state or combined state, depending on their chemical reactivity. The major steps to extract metals from ores include crushing, grinding, concentrating the ore, converting it to an oxide, extracting the crude metal, and refining the metal.
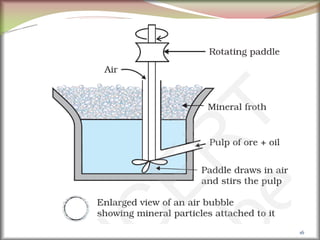
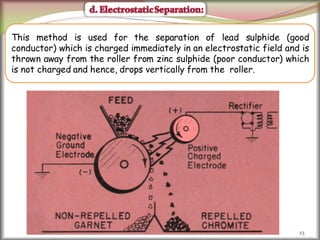
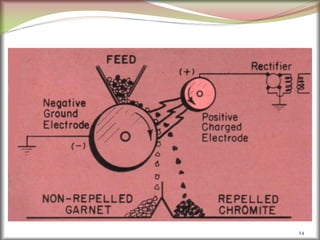
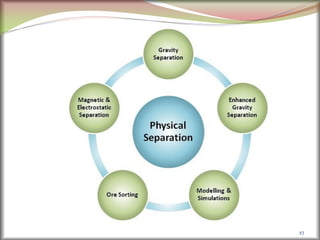
2. Concentrating the ore involves removing gangue and involves processes like magnetic separation, froth flotation, and electrostatic separation which separate materials based on differences in their physical properties.
3. Extractive metallurgy is the scientific process used to isolate metals from ores through various physical






![26
In moist air copper corrodes to produce a green layer on the surface.
What is that layer?
Copper, in the presence of moisture, oxygen and carbon dioxide of
atmosphere, is converted into a basic carbonate, called malachite of
composition,CuCo3.Cu(OH)2.This basic carbonate is deposited as
green layer on its surface.
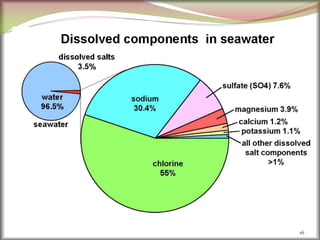
Metal sulphides occur mainly in rocks, but metal halides occur in lakes
and sea. Why?
Metal sulphides have high lattice energy and hence low solubility,
which can remain in rocks. [Sodium and potassium sulphides are
soluble in water so these do not occur in rocks.] But metal halides
have low lattice energy and are generally soluble in water and so
have dissolved out of the rocks and into the lakes/seas over time.](https://image.slidesharecdn.com/nonferrousextractivemetallurgy-231119145701-7929a293/85/Non-Ferrous-Extractive-Metallurgy-pdf-26-320.jpg)



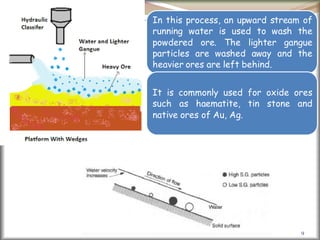
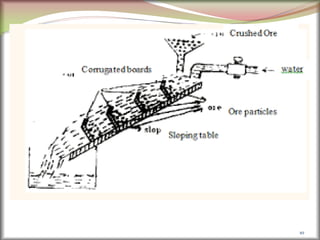
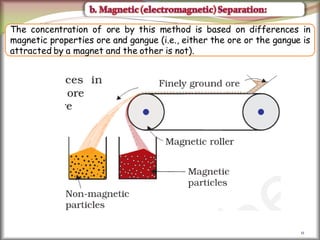

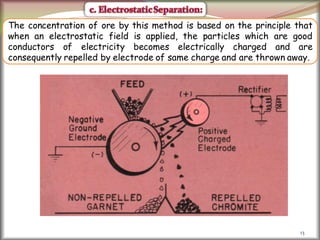












![41
In moist air copper corrodes to produce a green layer on the surface.
What is that layer?
Copper, in the presence of moisture, oxygen and carbon dioxide of
atmosphere, is converted into a basic carbonate, called malachite of
composition,CuCo3.Cu(OH)2.This basic carbonate is deposited as
green layer on its surface.
Metal sulphides occur mainly in rocks, but metal halides occur in lakes
and sea. Why?
Metal sulphides have high lattice energy and hence low solubility,
which can remain in rocks. [Sodium and potassium sulphides are
soluble in water so these do not occur in rocks.] But metal halides
have low lattice energy and are generally soluble in water and so
have dissolved out of the rocks and into the lakes/seas over time.](https://image.slidesharecdn.com/nonferrousextractivemetallurgy-231119145701-7929a293/85/Non-Ferrous-Extractive-Metallurgy-pdf-68-320.jpg)


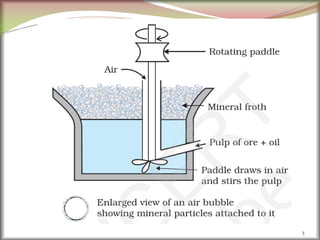

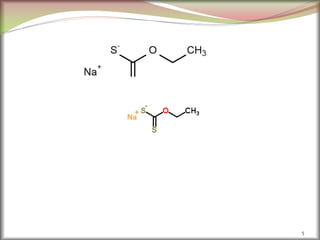


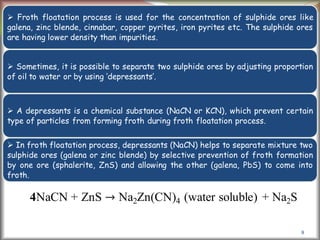








![6
As man learned to fashion weapons from iron and steel, copper began to assume another role.
Being a durable metal and possessed of great beauty, it was used extensively for household
utensils and water pipes and for marine uses and other purposes that required resistance to
corrosion. The unusual ability of this metal to conduct electric current accounts for its
greatest use today.
Property Details
Symbol Cu
Atomic number 29
Standard Atomic weight 63.546
Electronic Configuration [Ar] 3d¹⁰4s¹
Crystal structure FCC
Density 8.96 g/cc
Melting point 1084.32°C (1357.77K)
Boiling point 2562°C (2835K)
Heat of fusion 13.26 KJ/mol
Thermal conductivity 401 W/m K
Electrical conductivity 5.96×10¹⁰ S/m at 20°C
Poisson ration 0.34](https://image.slidesharecdn.com/nonferrousextractivemetallurgy-231119145701-7929a293/85/Non-Ferrous-Extractive-Metallurgy-pdf-86-320.jpg)
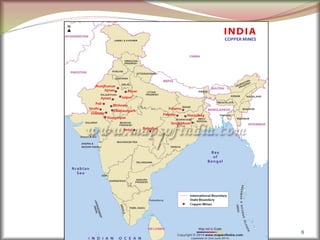






















![4
S.no
1. Symbol Zn
2. Atomic number 30
3. Abundancein the Earth crust 24
4. Electron configuration [Ar] 3d10
4s2
5. Crystalstructure HCP
6. Density 7.14 g/cc
7. Brinnel Hardness 412 MPa
8. Melting point 419.53
9. Electrical resistivity 6.0 × 10-8 Ω m
10. Thermal conductivity 116 W.m-1.k-1
11. Color White Silver Color
12. Price 175/- per kg](https://image.slidesharecdn.com/nonferrousextractivemetallurgy-231119145701-7929a293/85/Non-Ferrous-Extractive-Metallurgy-pdf-122-320.jpg)
![5
Name Chemical Formula
Sphalerite ZnS
Zincite ZnO
Franklinite
[ZnO(Fe, Mn)2O3]
Willemite Zn2SiO4
Smith Sonite ZnCO3
Location of ore in India : Zawar(Rajasthan),Sikkim,
Udhampur(jammu&kashmir)
Areas of extraction : ZawarMines (Rajasthan)
HZL(Hindustan Zinc Ltd)
COMINOCO-BINANI at Kerala](https://image.slidesharecdn.com/nonferrousextractivemetallurgy-231119145701-7929a293/85/Non-Ferrous-Extractive-Metallurgy-pdf-123-320.jpg)
















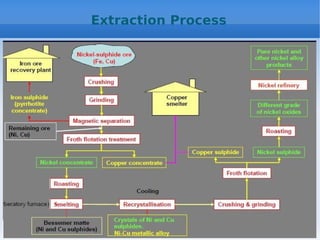



![Refining Process
1. Carbonyl Process for Refining Ni:
(i) Mond’s Process: In 1889, this refining process of Ni recovered by
Carl Langer and Ludwig Mond. In this process, at temperature (40-90)0 C
metallic Ni combine with CO to give gaseous nickel carbonyl [Ni(CO)4 ]. At
higher temperature (150-300)' C Ni(CO)4decomposes to give Ni and CO
gas.
Other forms of Carbonyls are volatile carbonyl [Fe(CO) 5 ],
Co carbonyl in tetracarbonyl [Co 2 (CO) 8 ] tricarbonyl [Co 4 (CO) 12 ] form.
Cu and other major elements are not form carbonyls.](https://image.slidesharecdn.com/nonferrousextractivemetallurgy-231119145701-7929a293/85/Non-Ferrous-Extractive-Metallurgy-pdf-155-320.jpg)



![2
S.no
1. Symbol Al
2. Atomic number 13
3. Abundancein the Earth crust 8.1%(firstabundantmetal)
4. Electron configuration [Ne]3s23p1
5. Crystalstructure FCC
6. Density 2.70 g/cc
7. Hardness 160-550MPa BHN
8. Melting point 660.32⁰C
9. Electrical conductivity 3.50*10⁷(S/mat20⁰C)
10. Thermal conductivity 237 W.m-1
.k-1
11. Color Silvery grey Color
12. Price 115/- per kg](https://image.slidesharecdn.com/nonferrousextractivemetallurgy-231119145701-7929a293/85/Non-Ferrous-Extractive-Metallurgy-pdf-160-320.jpg)





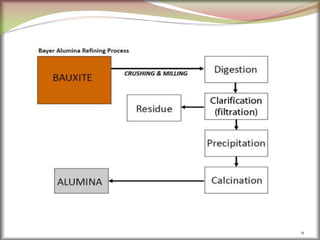



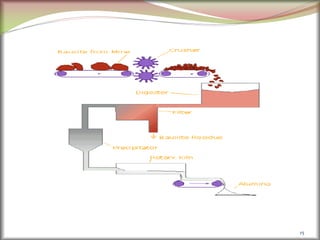



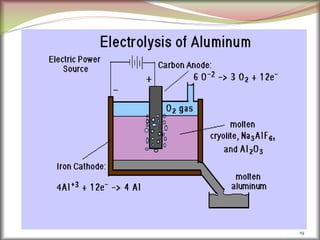

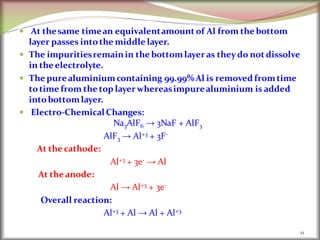
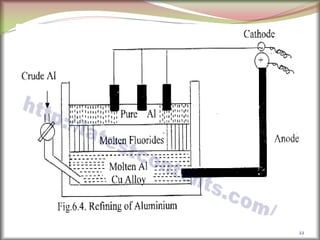

![2
Physical properties
Property Value
Symbol Pb
Atomic number 82
Standard Atomic weight 207.2
Electronic configuration [Xe]
Crystal structure FCC
Density at 20⁰C 11.34g/cm
Melting point 327°C
Boiling point 1755°C
Coefficient of thermal
expansion
29.1 μm/m•°C
Thermal conductivity 34.9 W/mK
Elastic modulus 16.8 GPa
Poisson ratio 0.42](https://image.slidesharecdn.com/nonferrousextractivemetallurgy-231119145701-7929a293/85/Non-Ferrous-Extractive-Metallurgy-pdf-182-320.jpg)

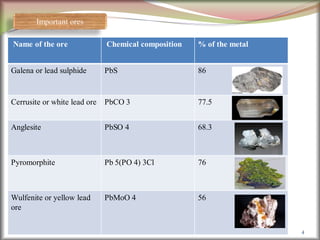


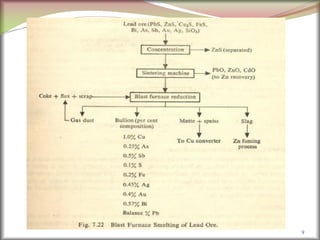



![Minerals of Mg:
NAME Chemical Formula
DOLOMITE CaCO3 MgCO3
MAGNESITE MgCO3
BRUCITE [Mg(OH)2]
CARNALLITE (MgCl2.KCl.XH2O)
EPSAM SALT (MgSO4.7H2O)](https://image.slidesharecdn.com/nonferrousextractivemetallurgy-231119145701-7929a293/85/Non-Ferrous-Extractive-Metallurgy-pdf-201-320.jpg)













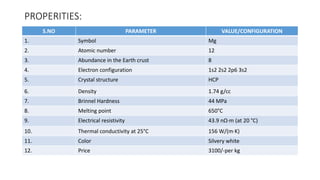
![S.no
1. Symbol U
2. Atomic number 92
3 Abundance in the Earth crust 51
4. Electron configuration [Rn] 5f3 6d1 7s2
5. Crystal structure Orthorhombic
6. Density 19.1 g/cm3
7. Thermal conductivity 27.5 W/(m·K)
9. Hardness 2350–3850 Mpa BHN
10. Melting point 1132.2 °C
11. color Silvery gray
12. Price 2,461.15 per kg](https://image.slidesharecdn.com/nonferrousextractivemetallurgy-231119145701-7929a293/85/Non-Ferrous-Extractive-Metallurgy-pdf-220-320.jpg)











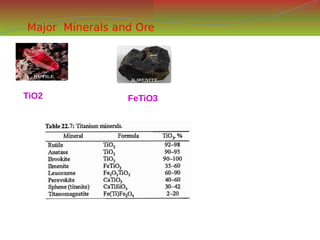

![S.no Property Data
Symbol Ti
Atomic number 22
Standard atomic weight 47.867
Abundance in the Earth crust as
an element
9
Electron configuration [Ar] 3d²4s²
Crystal structure HCP
Density at 20°C 4.506 g/cm3
Thermal conductivity 21.9 W/(m·K)
Electrical resistivity 420 nΩ·m (at 20 °C)
Hardness 716–2770 MPa BHN
Melting point 1668°C,(3034°F)
Boiling point (3287 °C)
color Silvery-white
metallic
Price ---- per kg](https://image.slidesharecdn.com/nonferrousextractivemetallurgy-231119145701-7929a293/85/Non-Ferrous-Extractive-Metallurgy-pdf-237-320.jpg)






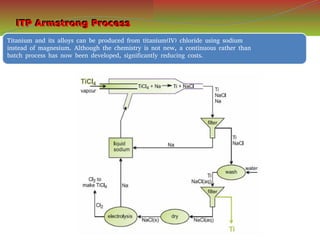

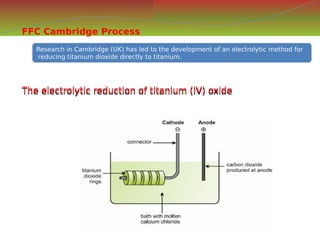




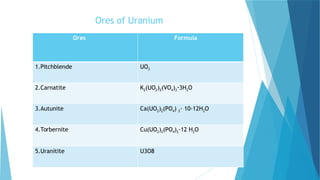
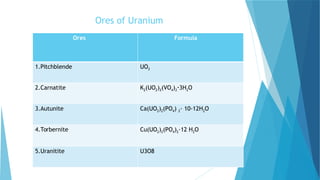
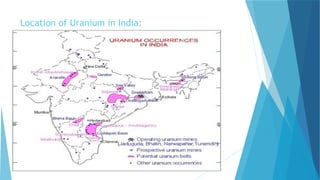
![S.no
1. Symbol U
2. Atomic number 92
3 Abundance in the Earth crust 51
4. Electron configuration [Rn] 5f3 6d1 7s2
5. Crystal structure Orthorhombic
6. Density 19.1 g/cm3
7. Thermal conductivity 27.5 W/(m·K)
9. Hardness 2350–3850 Mpa BHN
10. Melting point 1132.2 °C
11. color Silvery gray
12. Price 2,461.15 per kg](https://image.slidesharecdn.com/nonferrousextractivemetallurgy-231119145701-7929a293/85/Non-Ferrous-Extractive-Metallurgy-pdf-257-320.jpg)