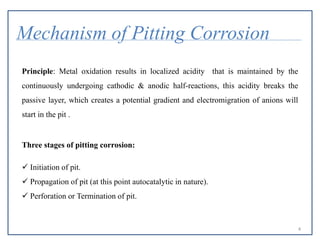
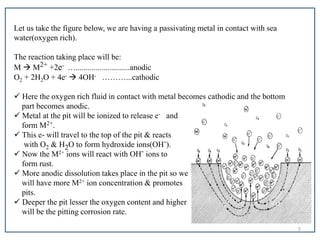
Pitting corrosion is a localized form of corrosion that leads to the formation of small cavities or holes in the material. It occurs when small areas become active (anodic) while the surrounding areas remain passive (cathodic). This creates galvanic cells that drive the corrosion process. Pitting corrosion initiates at defects on the material surface and then propagates in an autocatalytic manner. It is most common in alloys protected by a passive film when exposed to environments containing chlorides, oxygen, and stagnant conditions. Proper material selection, surface finishing, controlling environmental factors like pH and chloride levels, and using protective coatings or cathodic protection can help prevent pitting corrosion.