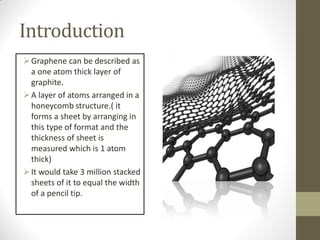
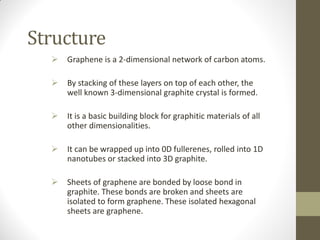
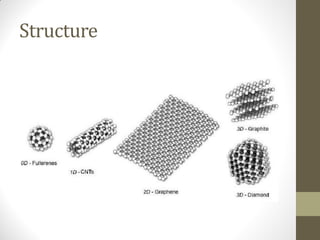
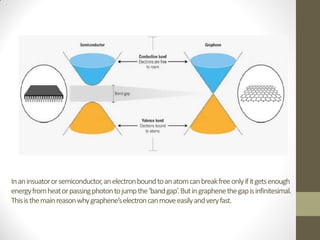
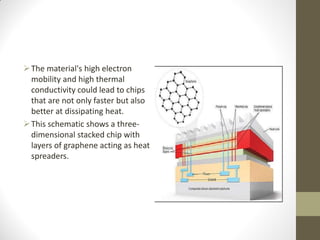
Graphene is a single layer of carbon atoms arranged in a honeycomb lattice. It is the strongest material ever tested, very light and highly conductive. Graphene was first isolated in 2004 and has potential applications in electronics, solar cells, and composite materials due to its unique properties. Some key properties include high strength, transparency, conductivity, flexibility and thermal conductivity. Graphene could be used to make faster computer chips, transparent touchscreens, and flexible electronics.