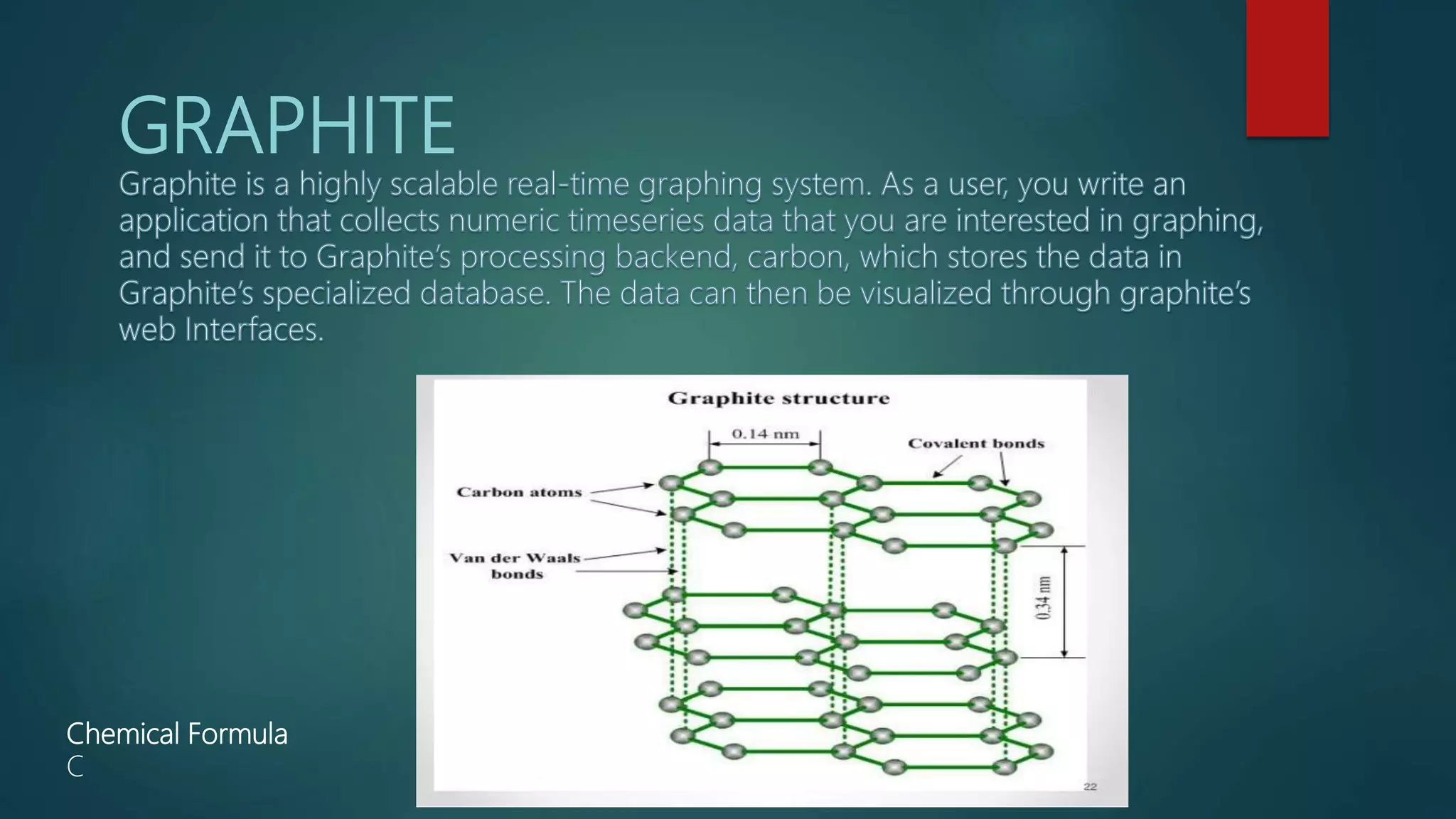
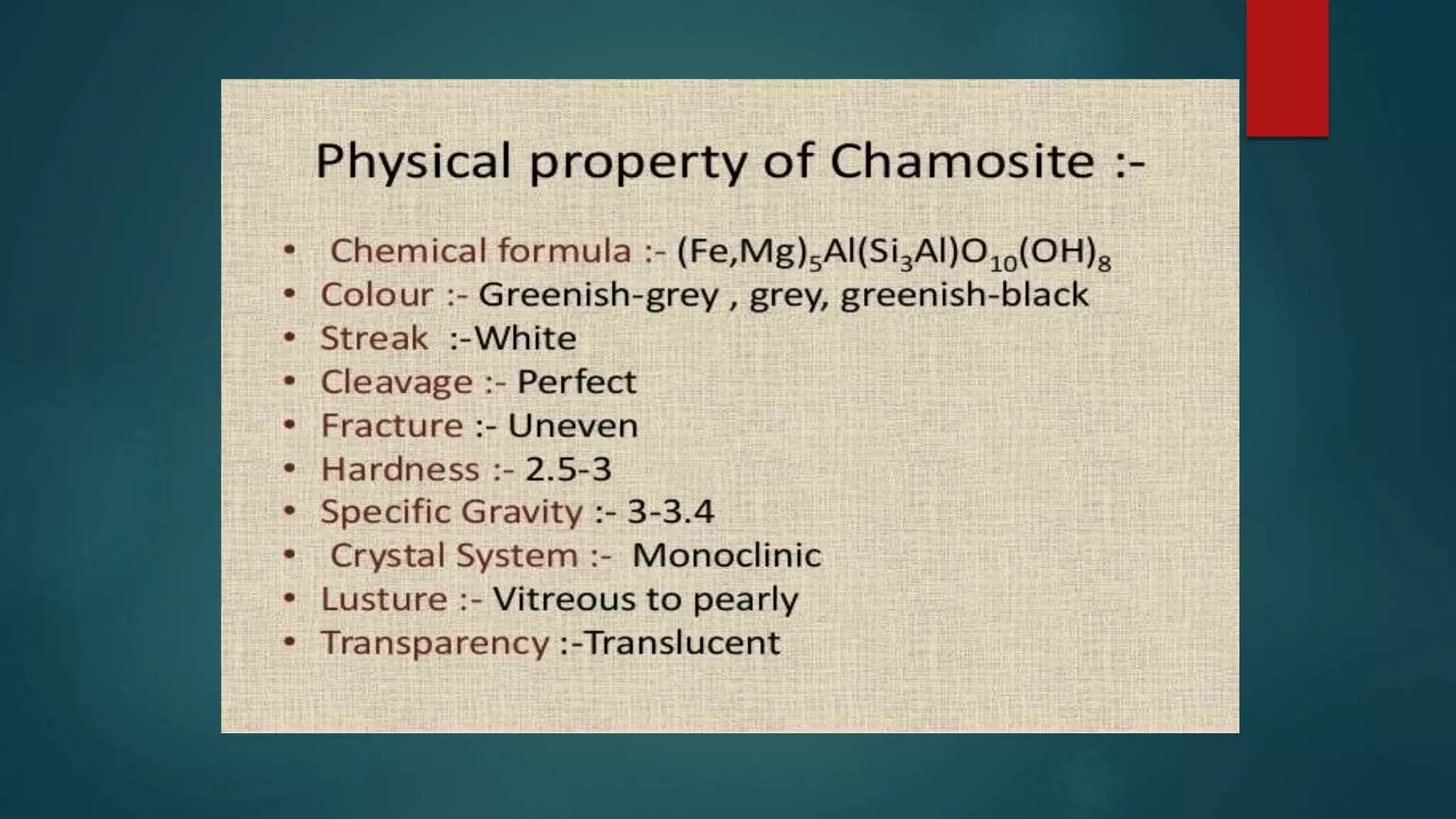
The document outlines the classifications and properties of graphite, detailing natural and synthetic types, their extraction methods, and various applications in industries such as chemicals, nuclear, and electronics. It also discusses marble, describing its origin, composition, uses in construction, and its susceptibility to acidic agents. Both materials are highlighted for their significant industrial and artistic roles.