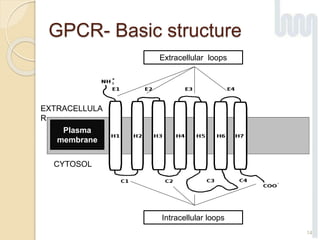
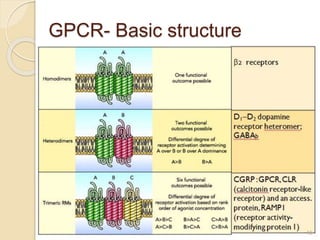
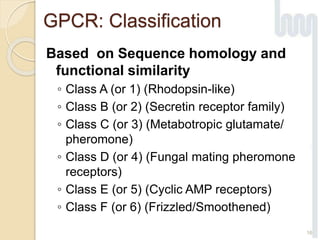
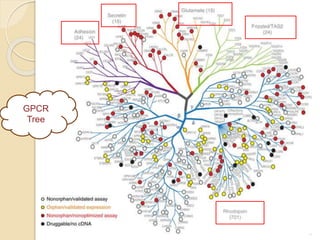
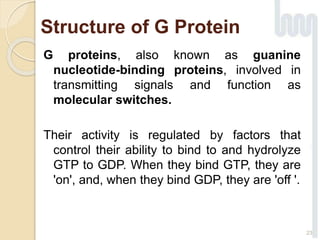
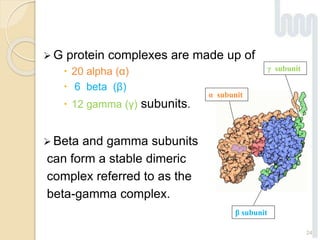
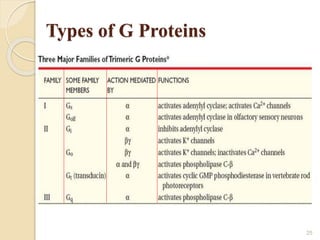
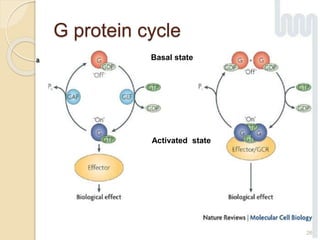
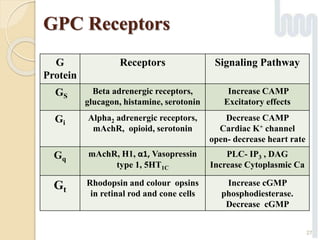
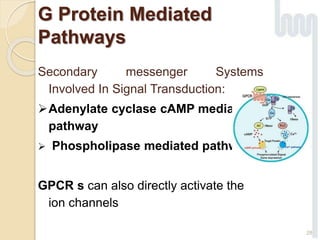
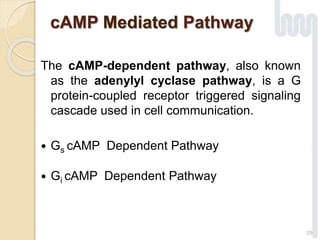
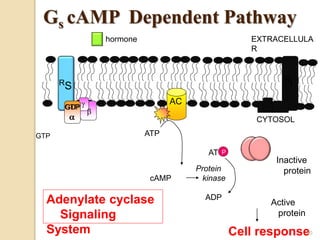
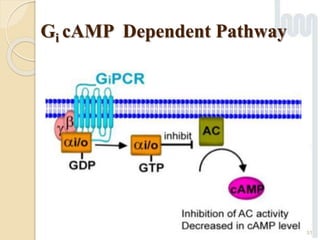
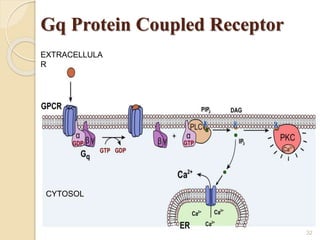
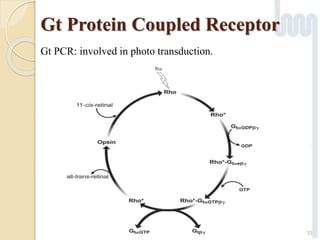
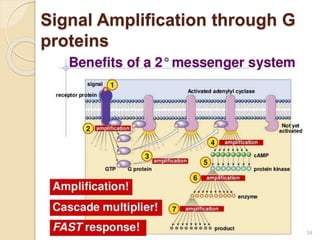
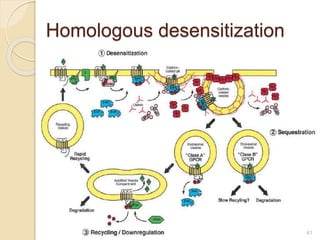
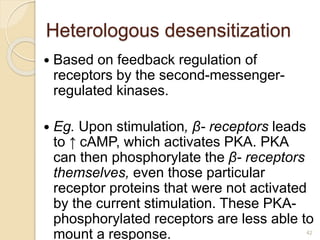
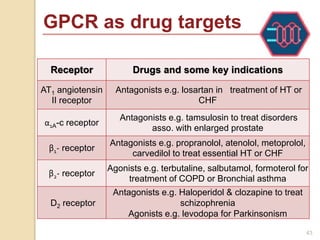
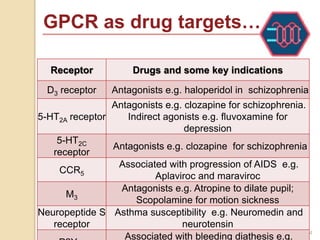
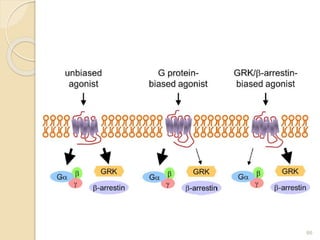
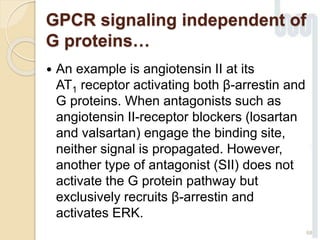
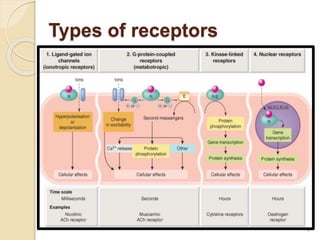
The document provides an overview of g-protein-coupled receptors (GPCRs), detailing their structure, classification, physiological roles, and significance as drug targets. It highlights GPCRs' involvement in various biological functions and their connection to multiple diseases, as well as the challenges in studying orphan GPCRs—where the natural ligands remain unknown. Recent developments in GPCR research, including ligand-induced selective signaling, are also discussed, emphasizing their potential for targeted drug development.





![The importance of GPCRs
1. Number (C.elegans 1100; H. sapiens, ~1000;
D. melanogaster, 160; reflects number of
olfactory receptor genes in worm [~1000] and
mammal [several hundreds]), a few % of
genome; 300-400 non-olfactory GPCRs)
2. Diversity (mostly small molecule ligands)
3. Evolutionarily conserved yeast to man (yeast
Ga 45% identical to mammalian Gia)
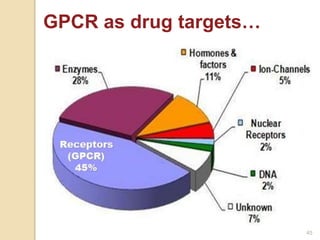
4. Pharmaceutical importance: ~500 known
molecular targets of drugs, 60% of these are
cell surface receptors, 75% of these are
GPCRs (GPCRs = ~45% of all known drug
targets) 9](https://image.slidesharecdn.com/gpcr-160211124029/85/G-Protein-Coupled-Receptors-9-320.jpg)