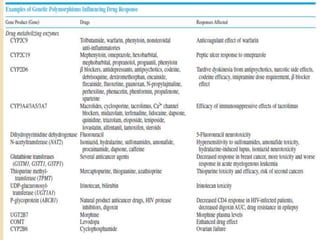
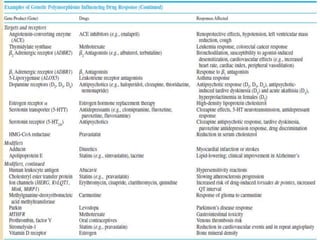
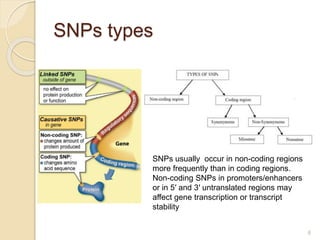
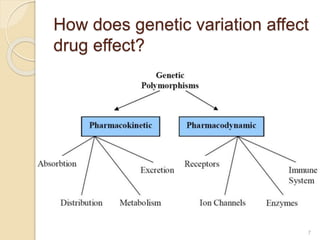
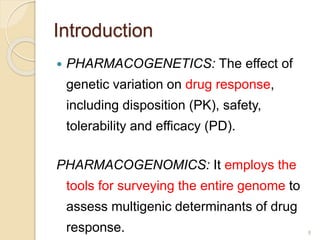
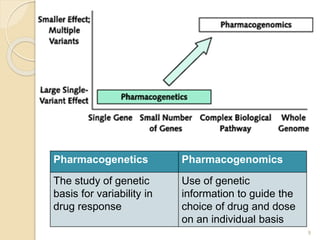
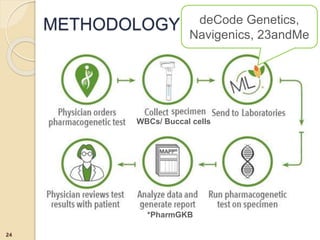
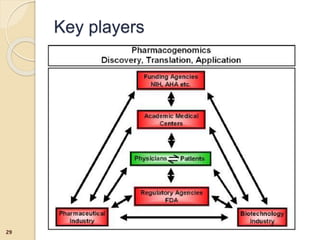
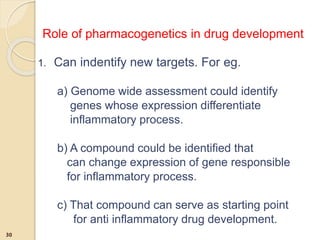
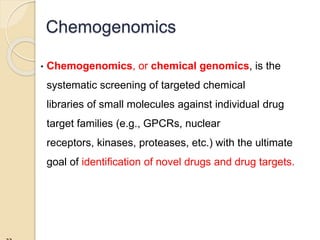
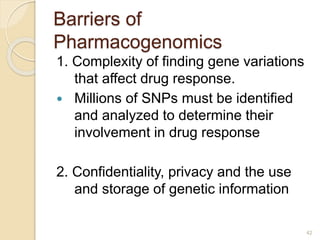
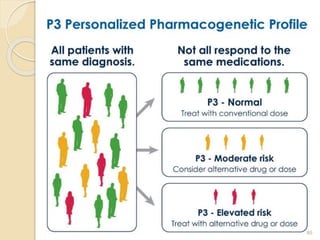
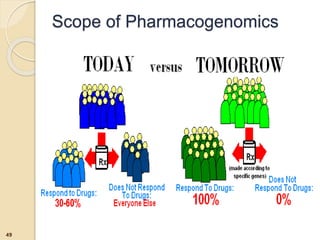
The document discusses pharmacogenetics and pharmacogenomics, emphasizing the role of genetic variations in drug response and the potential for personalized medicine. It highlights the importance of pharmacogenetic testing in improving drug efficacy and minimizing adverse effects, while also addressing the barriers to implementation in clinical practice. The text outlines methodologies for pharmacogenomic tests and explores the implications for drug development and patient care.




















![References
51
[1]Relling MV, Giacomini KM. Pharmacogenetics Brunton
Laurence, Chabner Bruce, Knollman Bjorn, editors.
Goodman and Gillman’s The Pharmacological Basis of
Theraputics.12ed. USA: McGraw Hills; 2011.p145-68.
[2] Rang HP, Dale M M, Ritter JM, Flower RJ, Henderson
G. Pharmacogenetics, Pharmacogenomics &
Personalised medicine. Hyde Madelane, Mortimer
Alexandra, editors. Pharmacology. 7ed.Britain: Elsevier
Churchill Living stone ;2012.p132-8.
[3] http://www.genome.gov/10002077#al-2
[4]http://www.fda.gov/drugs/scienceresearch/researcharea
s/pharmacogenetics/ucm083378.htm](https://image.slidesharecdn.com/my9thseminar-160702062544/85/Pharmacogenetics-and-Pharmacogenomics-50-320.jpg)
![References…
[5]http://www.fda.gov/downloads/regulatoryinformat
ion/guidances/ucm126957.pdf
[6]http://www.fda.gov/downloads/Drugs/ScienceRe
search/ResearchAreas/Pharmacogenetics/ucm1
16702.pdf
[7] Semizarov D, Blomme D.Introduction genomics
& personalised medicine.Genomics in Drug
Discovery and Development .1ed. USA: Wiley;
2009.p1-24.
[8] Dr. Hemant Banga’s Seminar on
Pharmacogenetics
52](https://image.slidesharecdn.com/my9thseminar-160702062544/85/Pharmacogenetics-and-Pharmacogenomics-51-320.jpg)