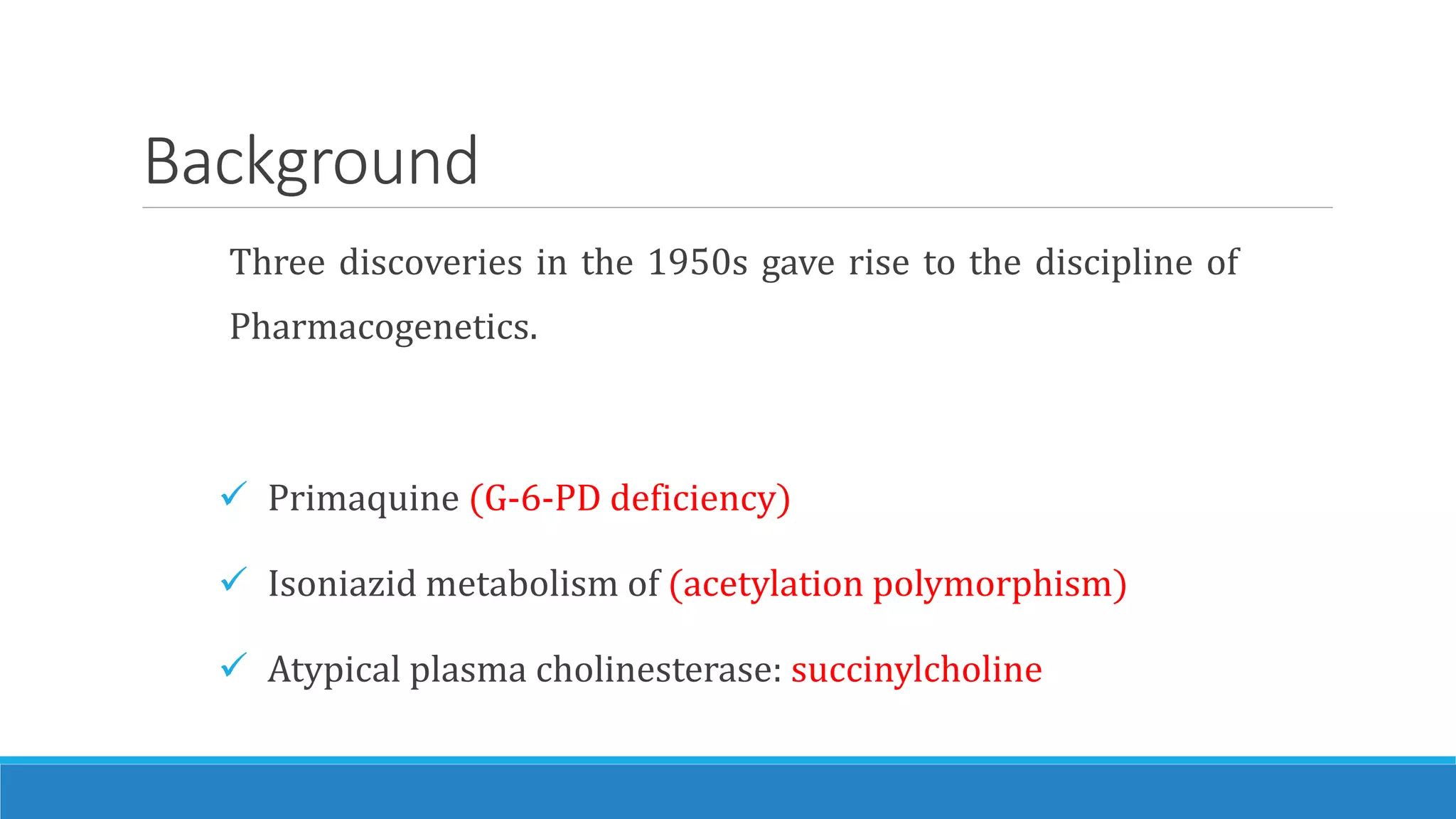
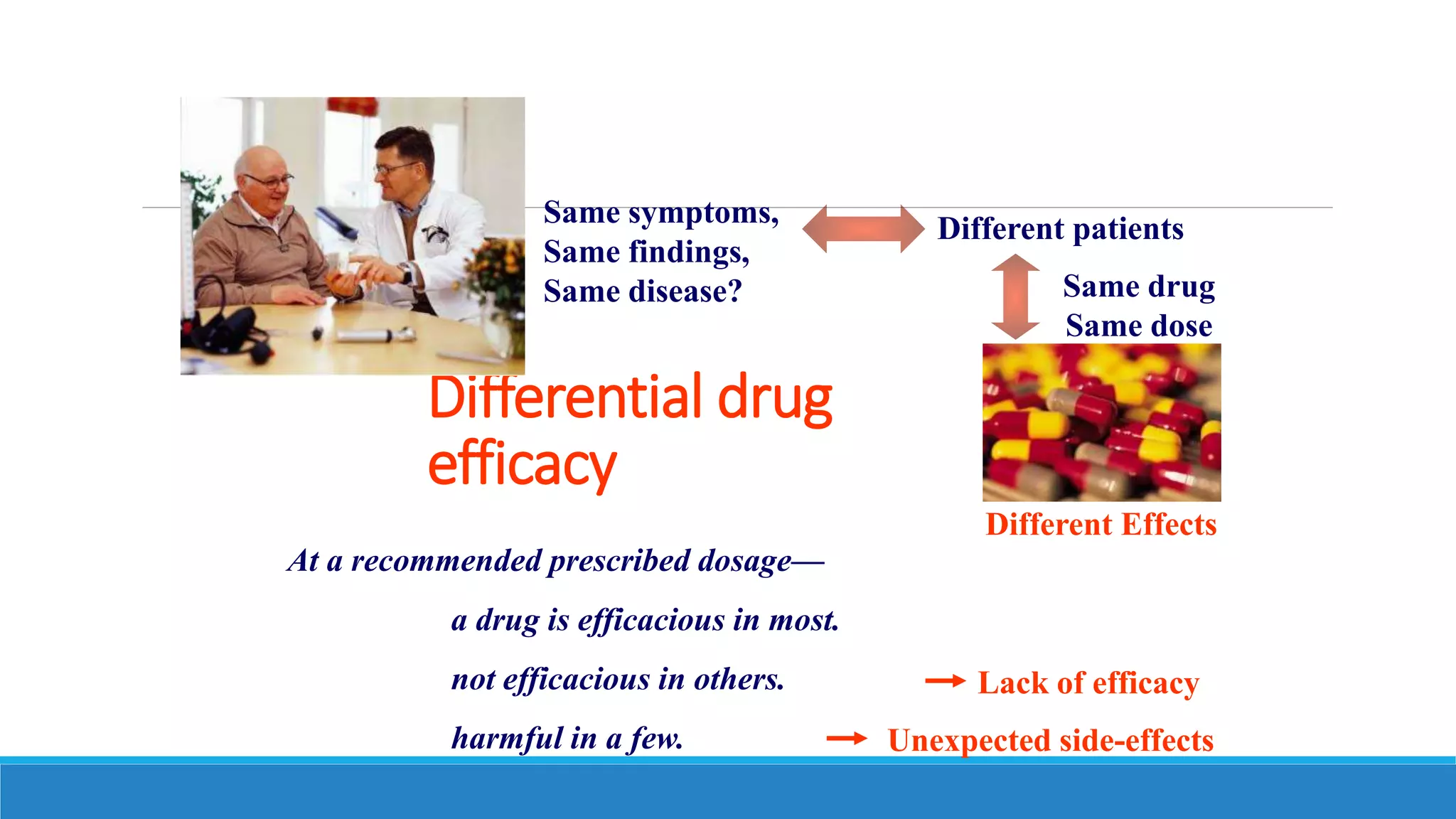
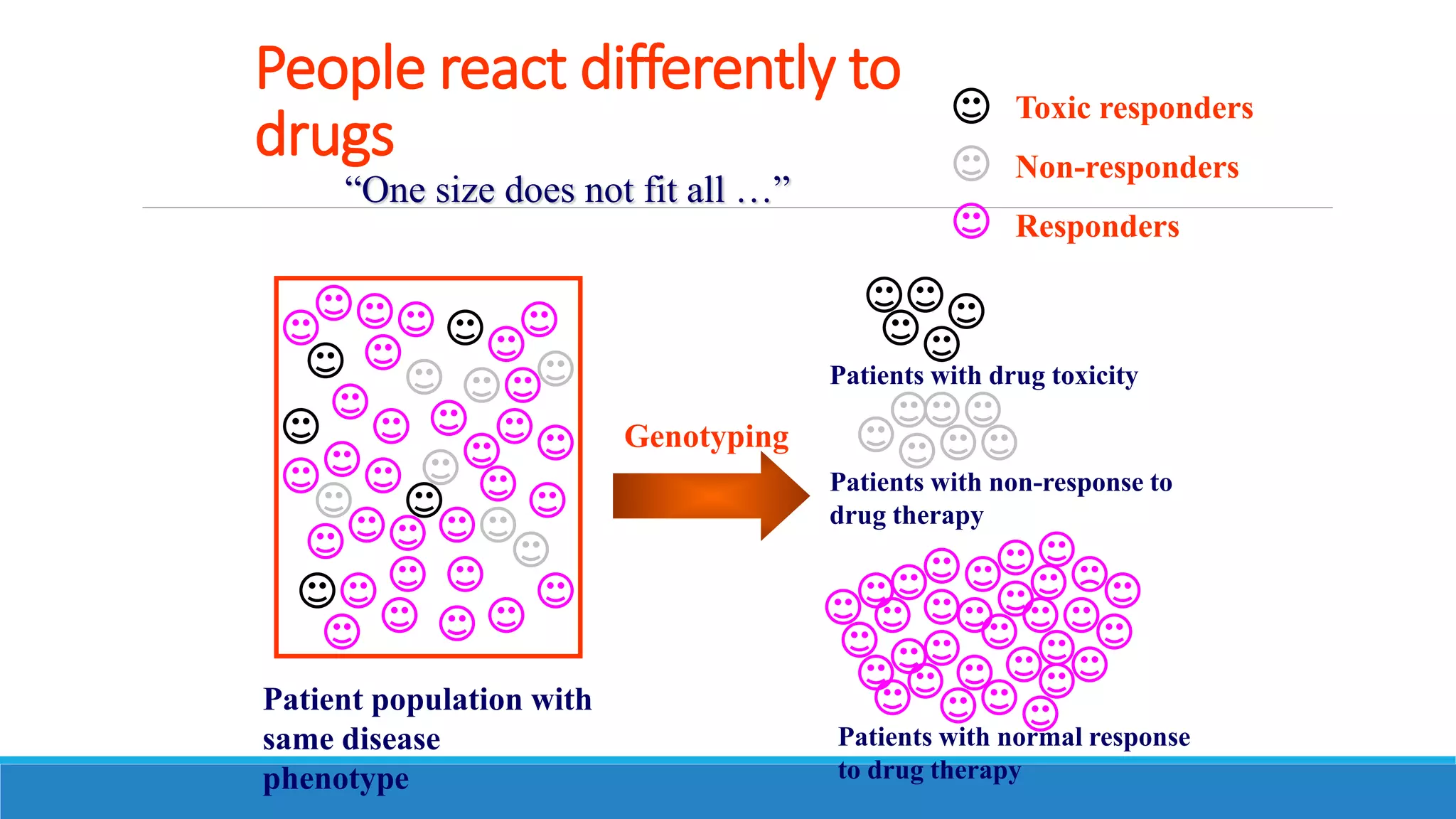
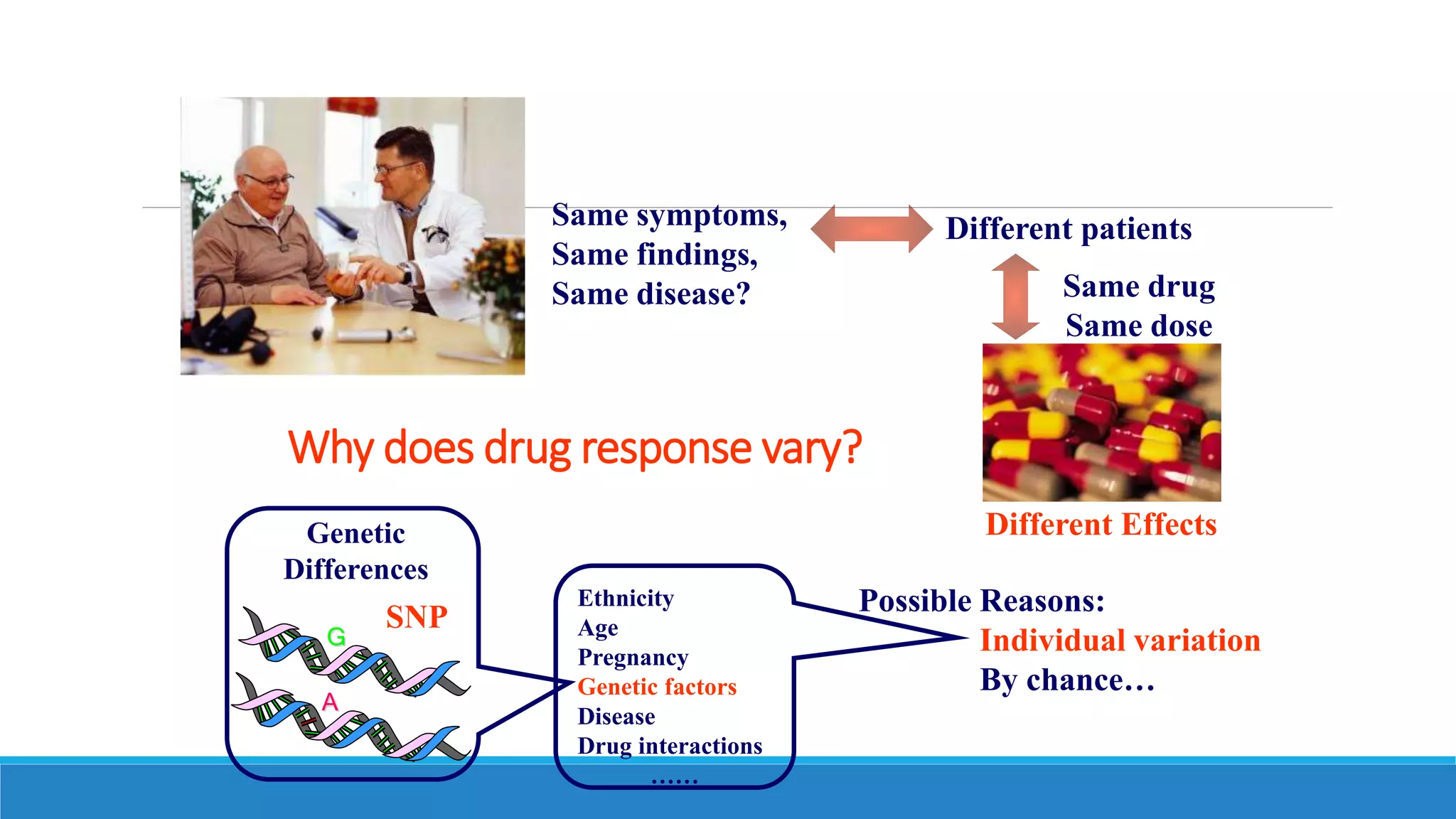
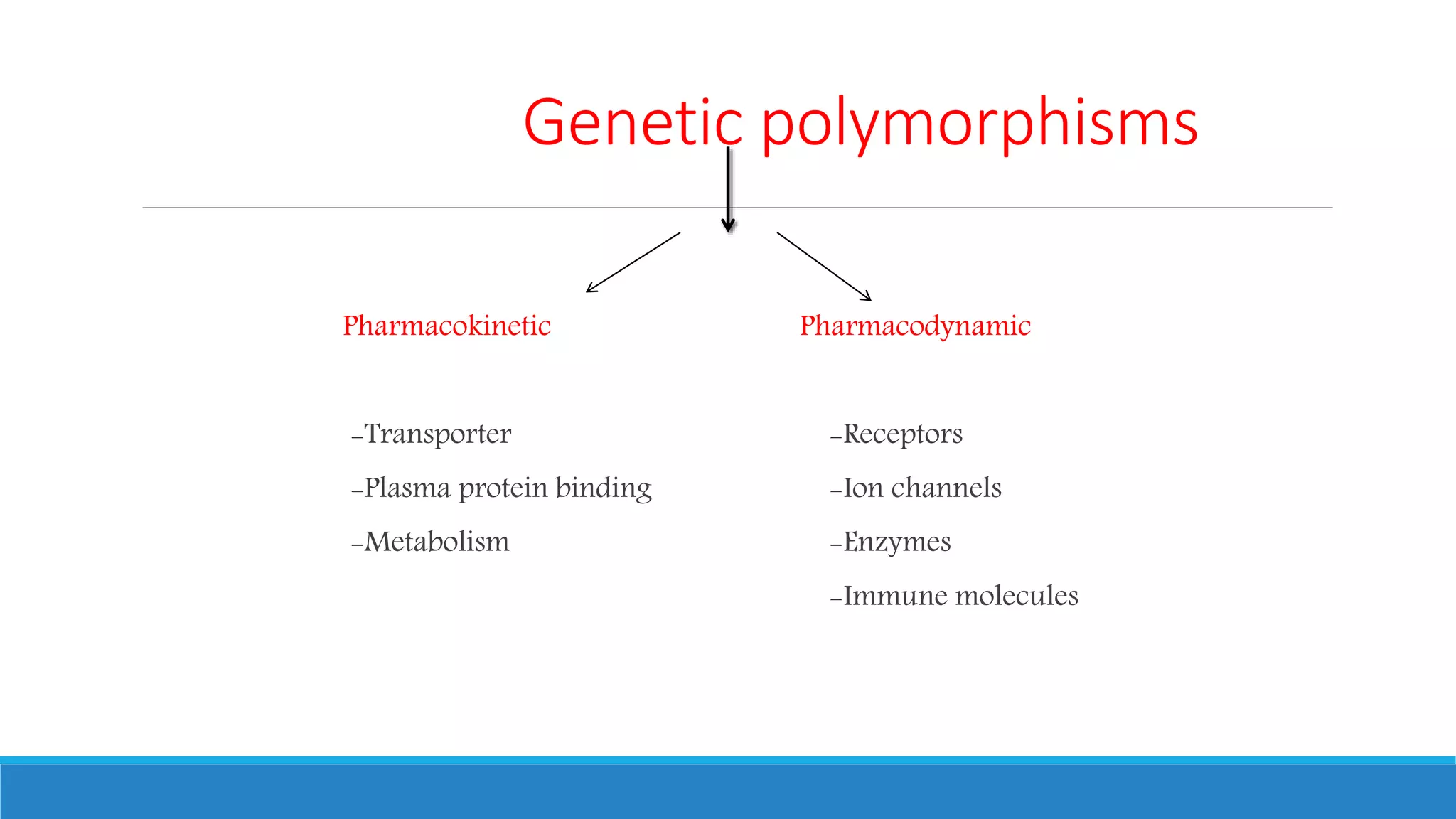
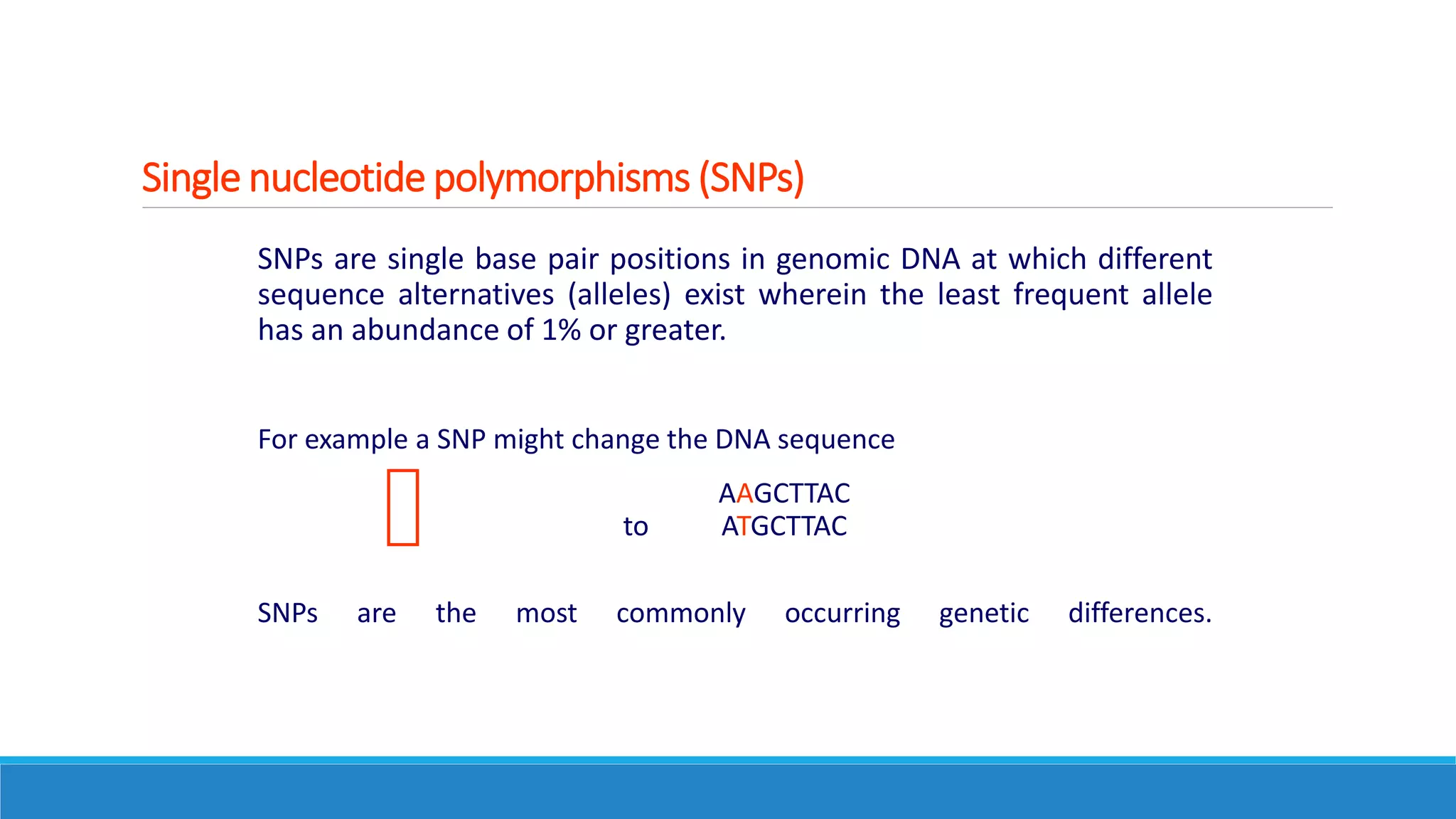
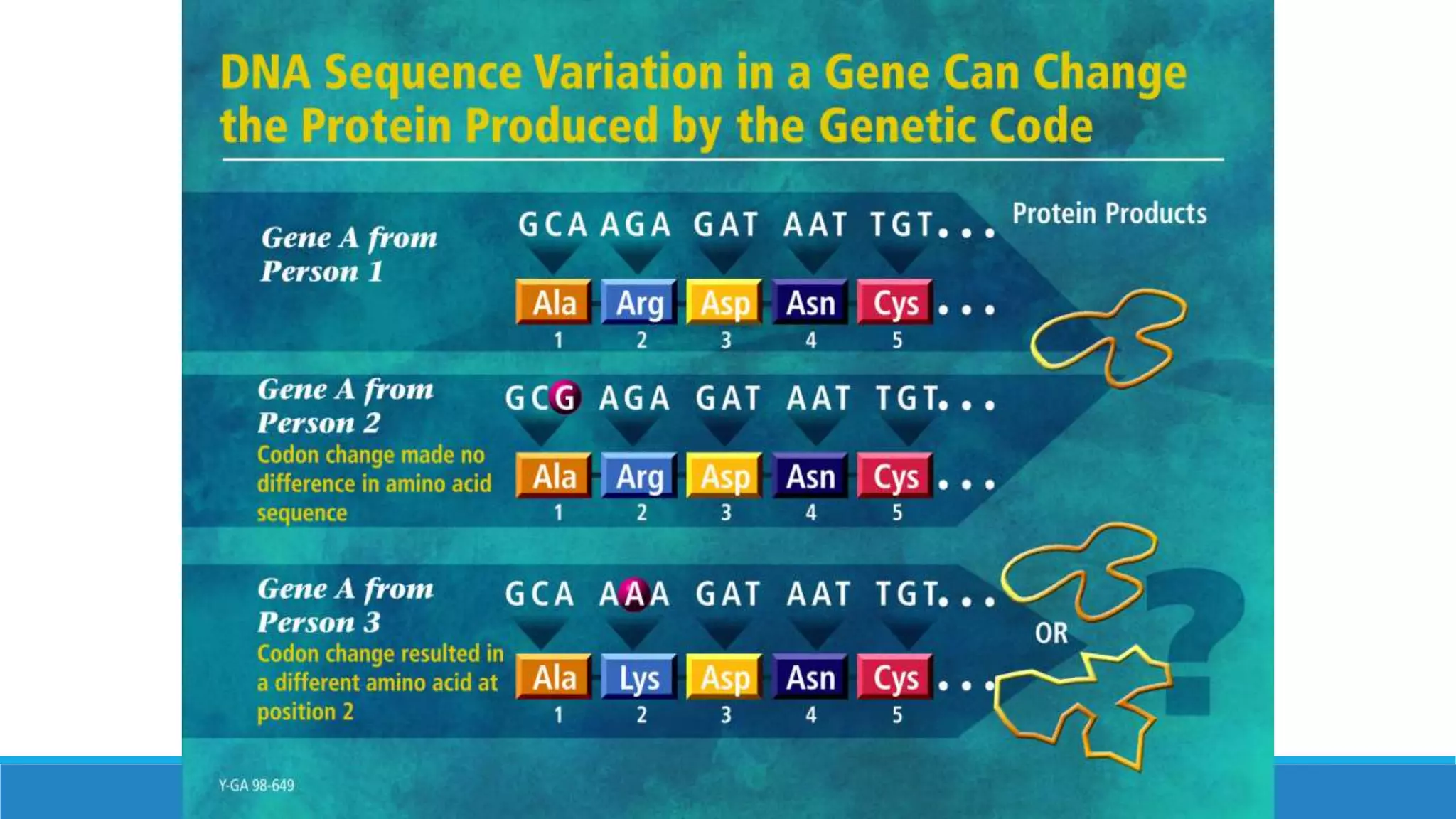
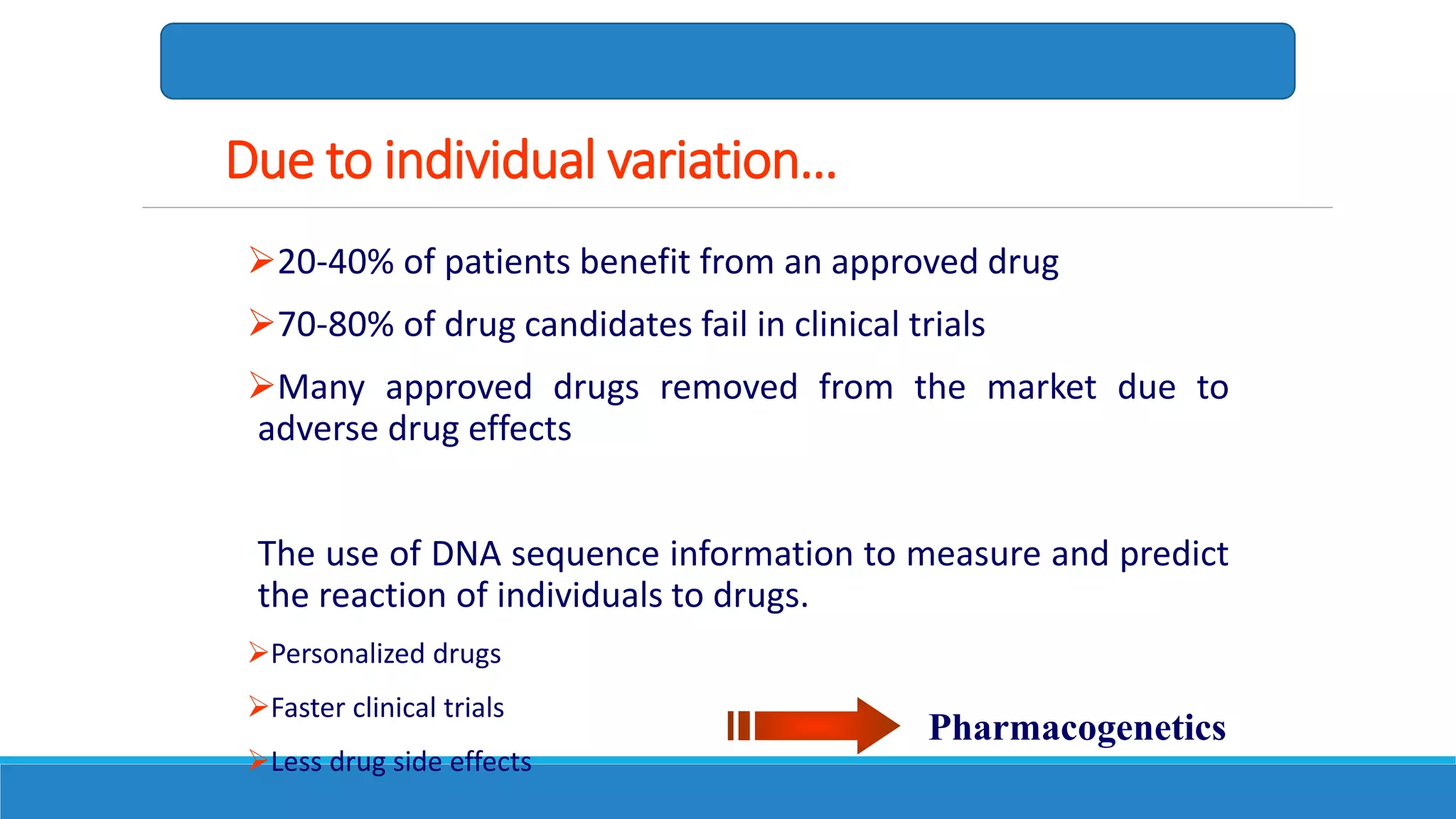
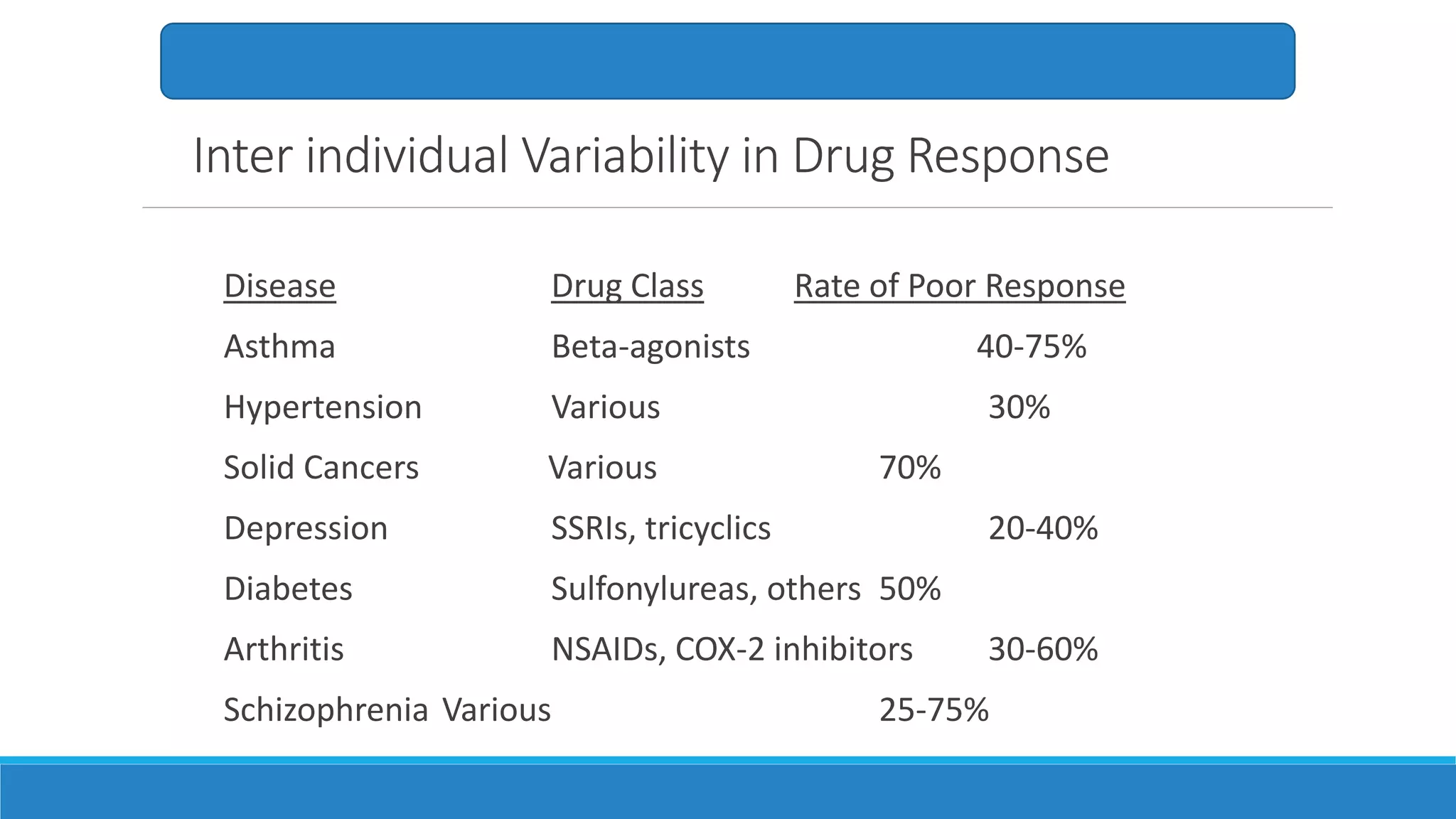
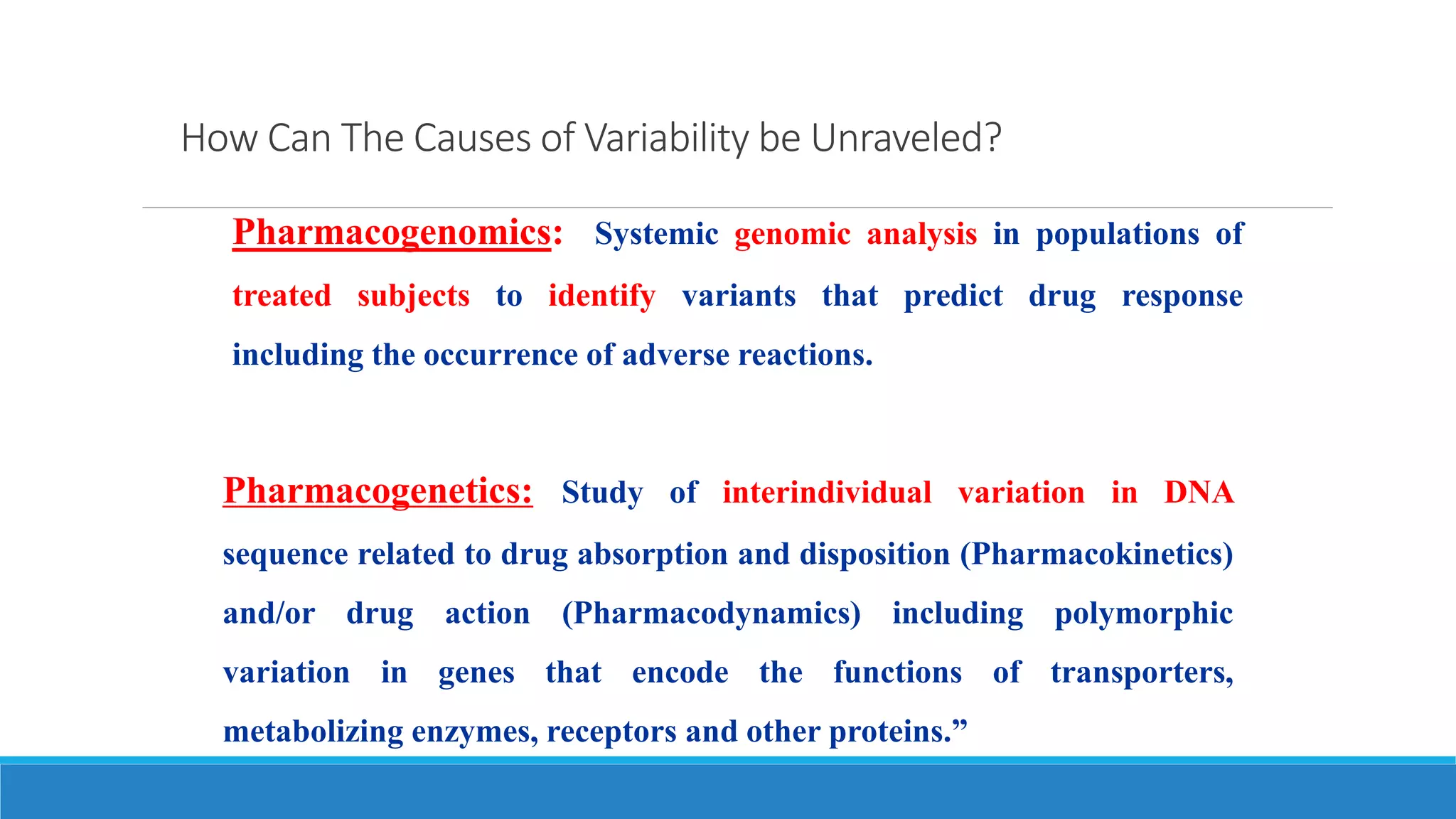
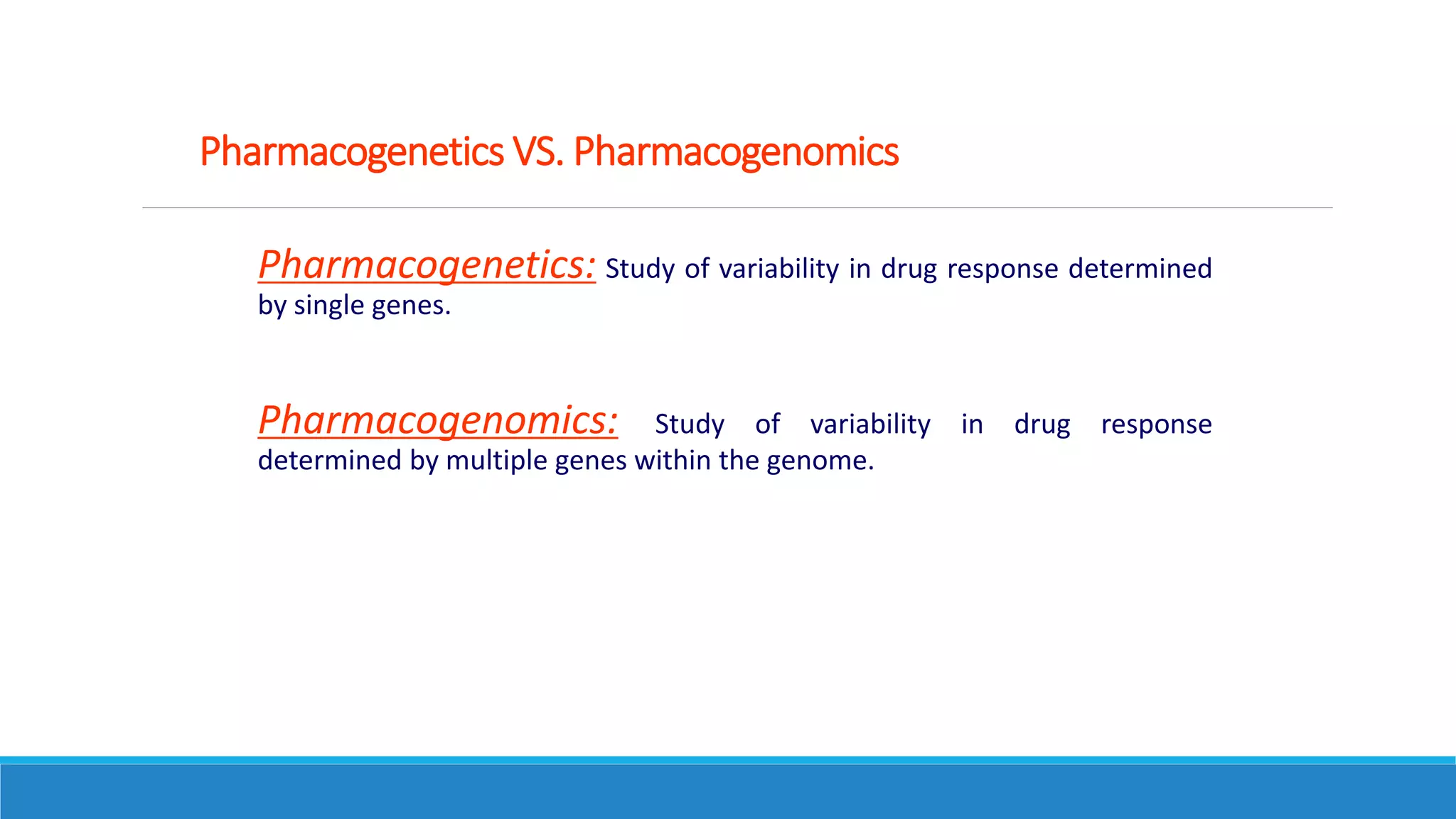
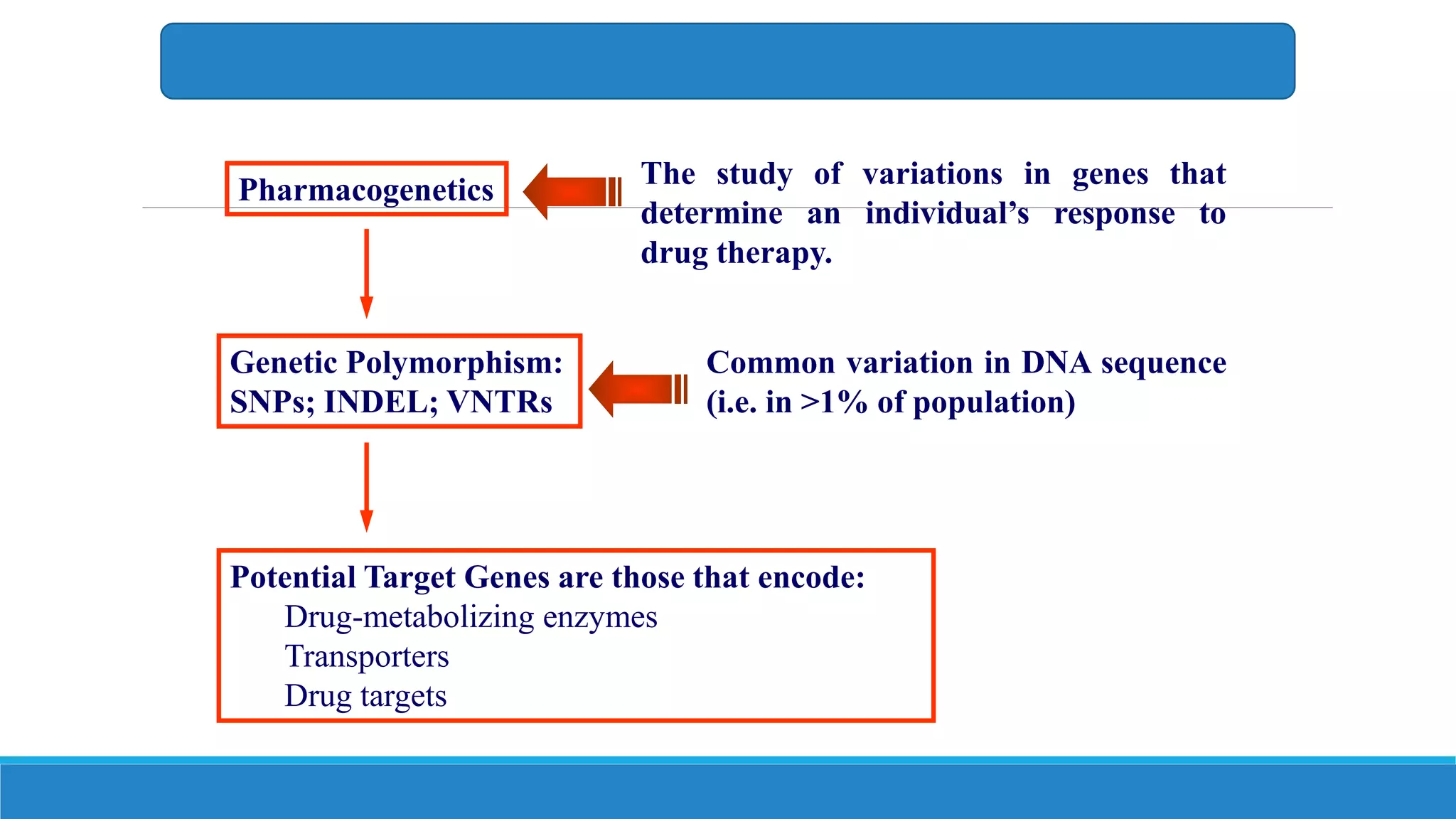
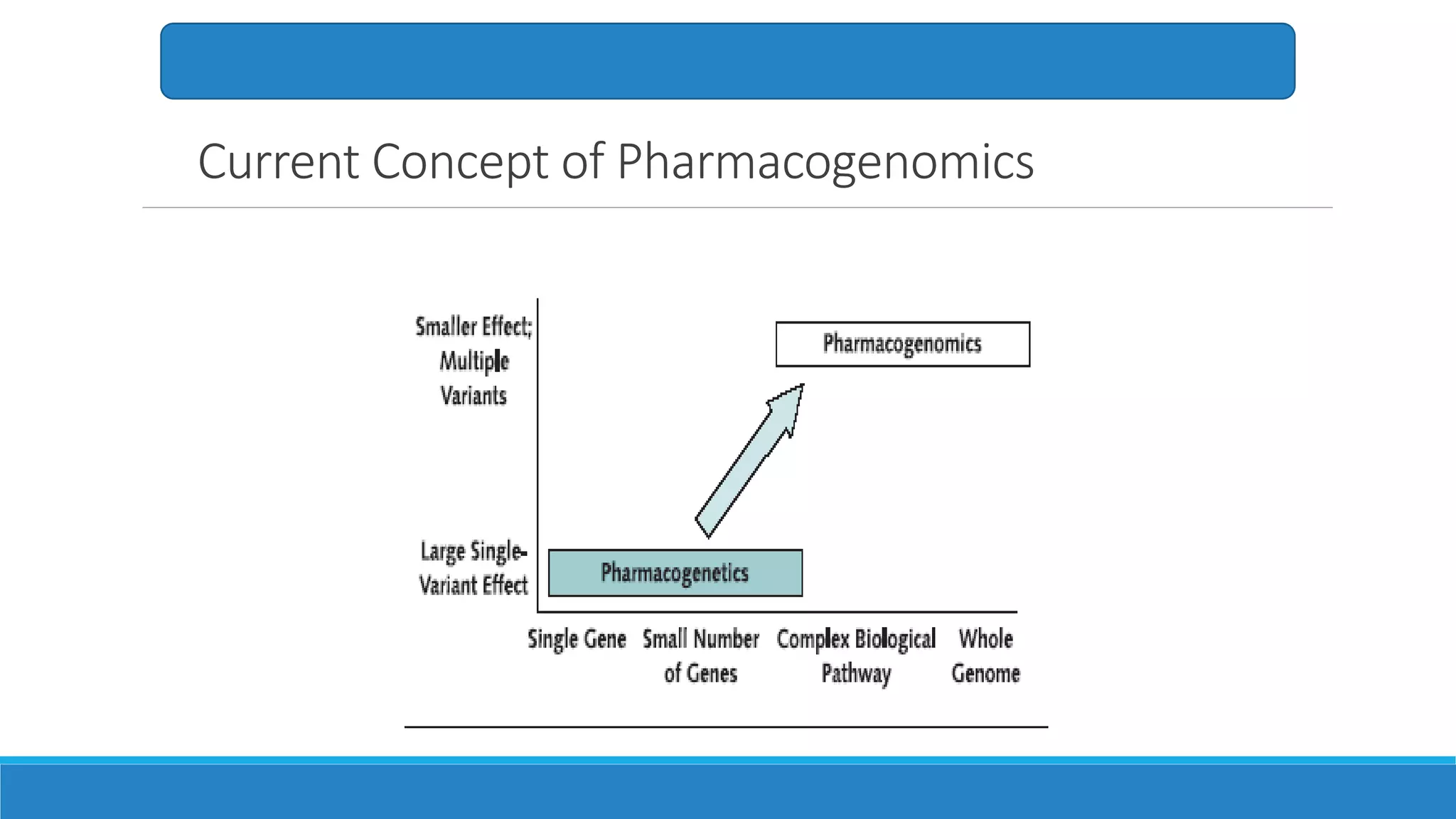
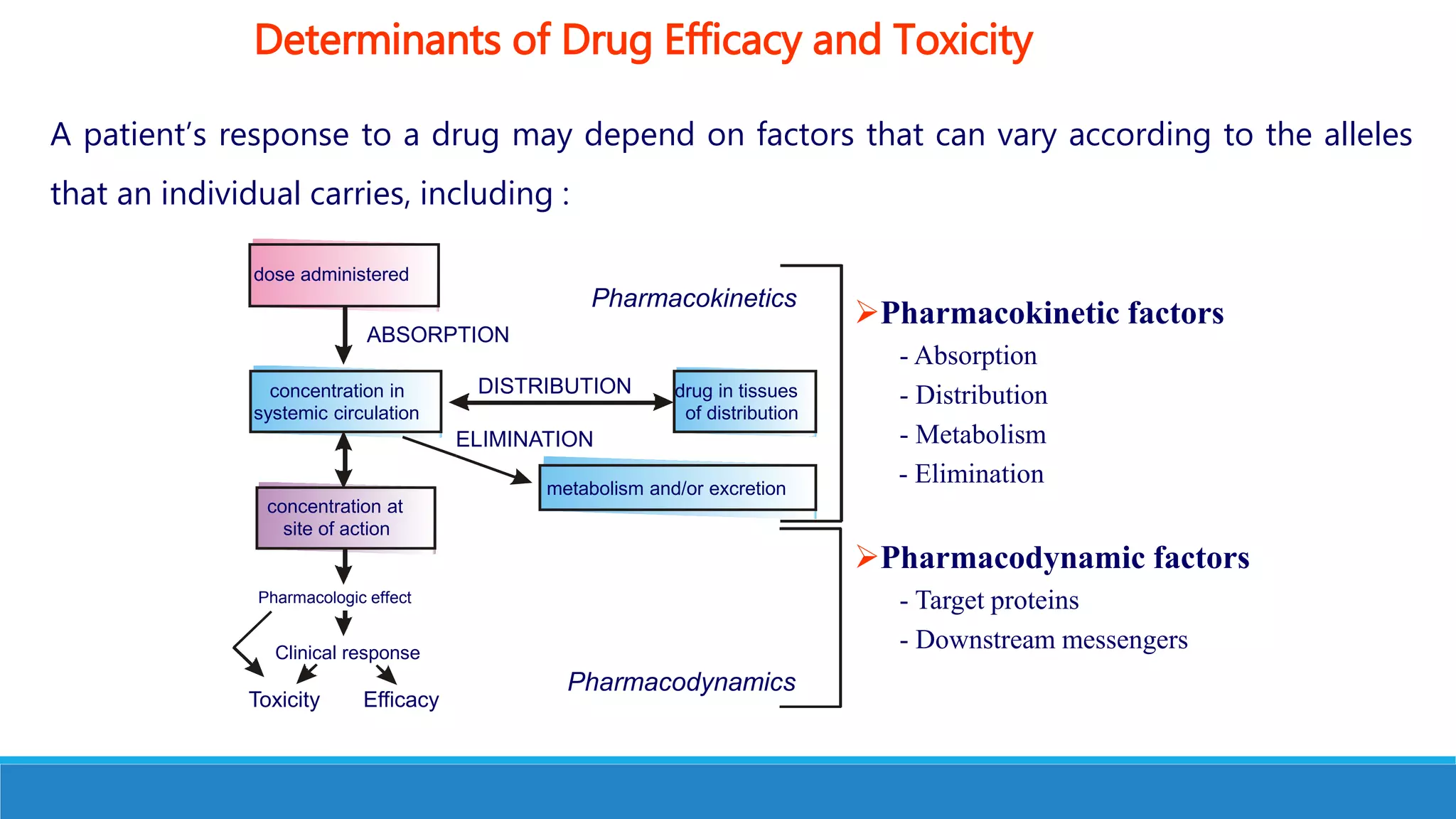
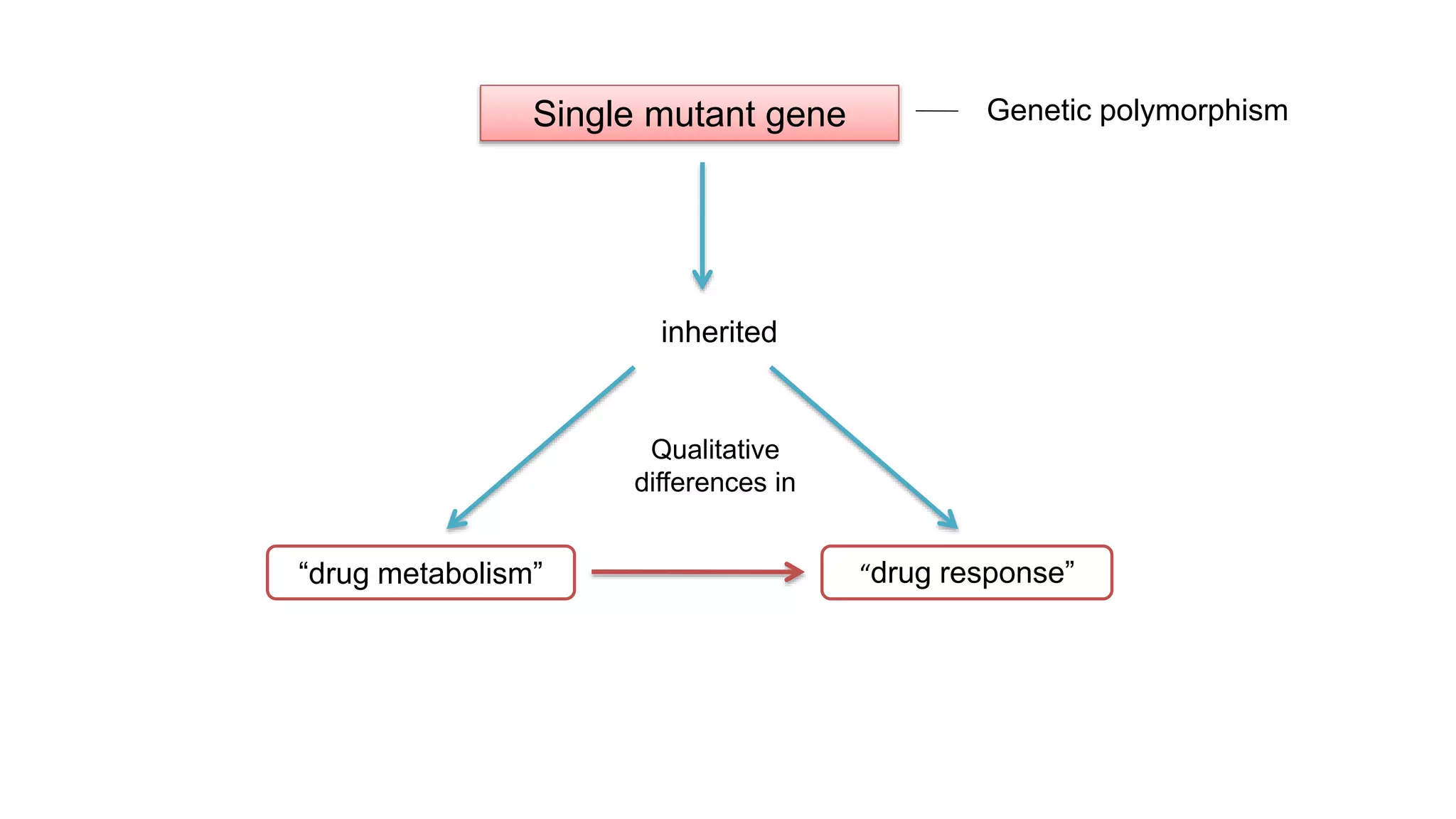
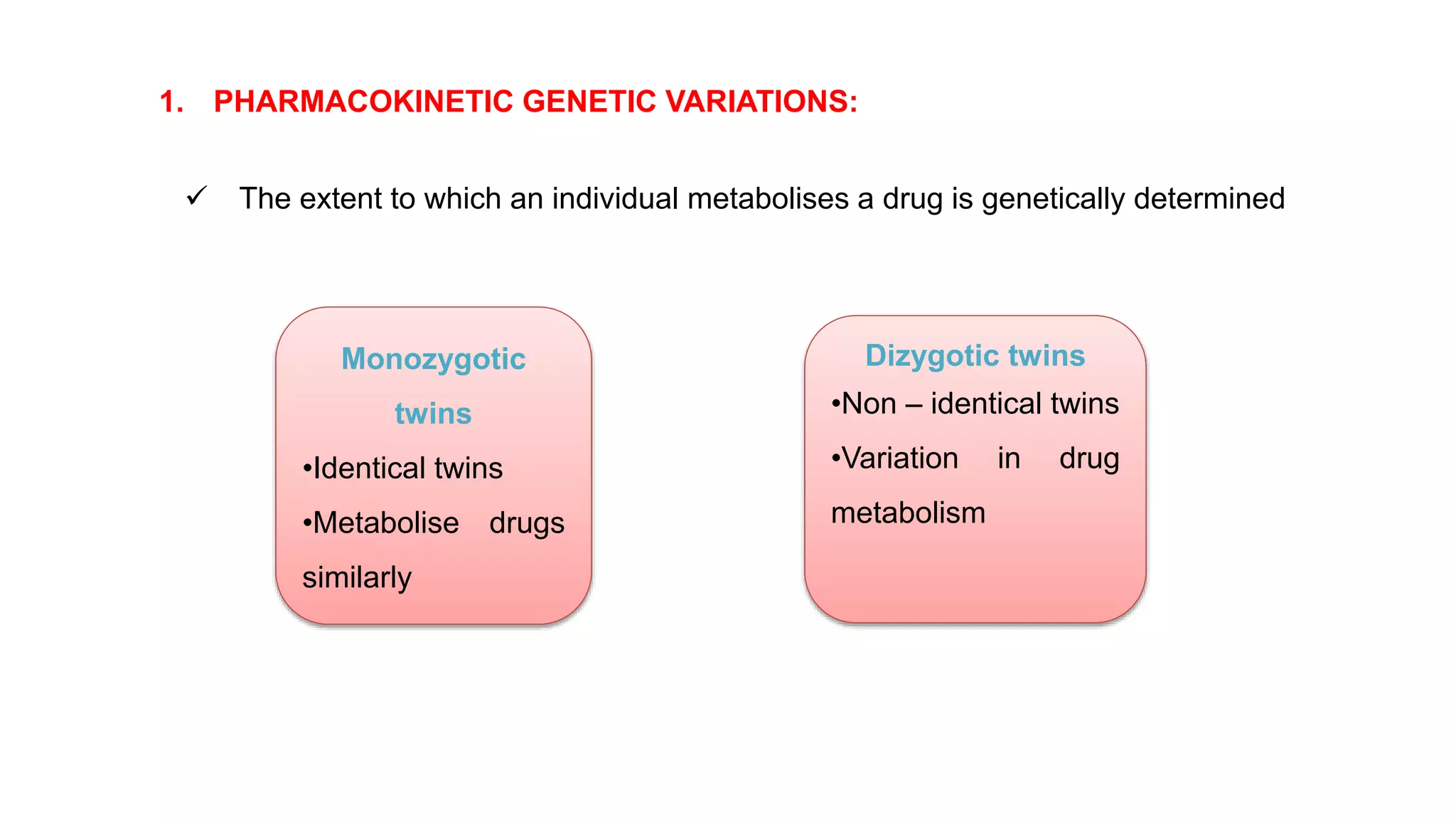
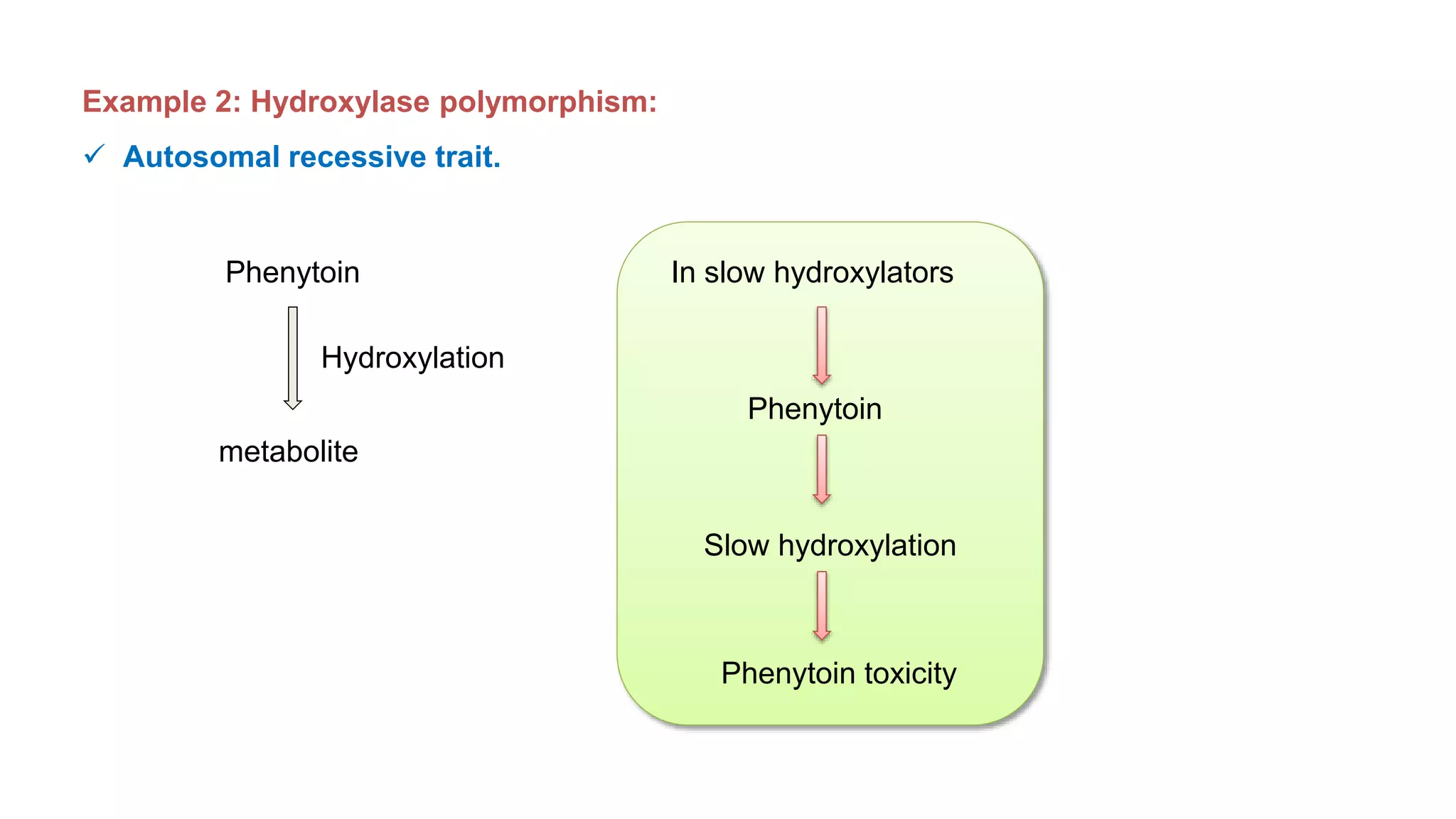
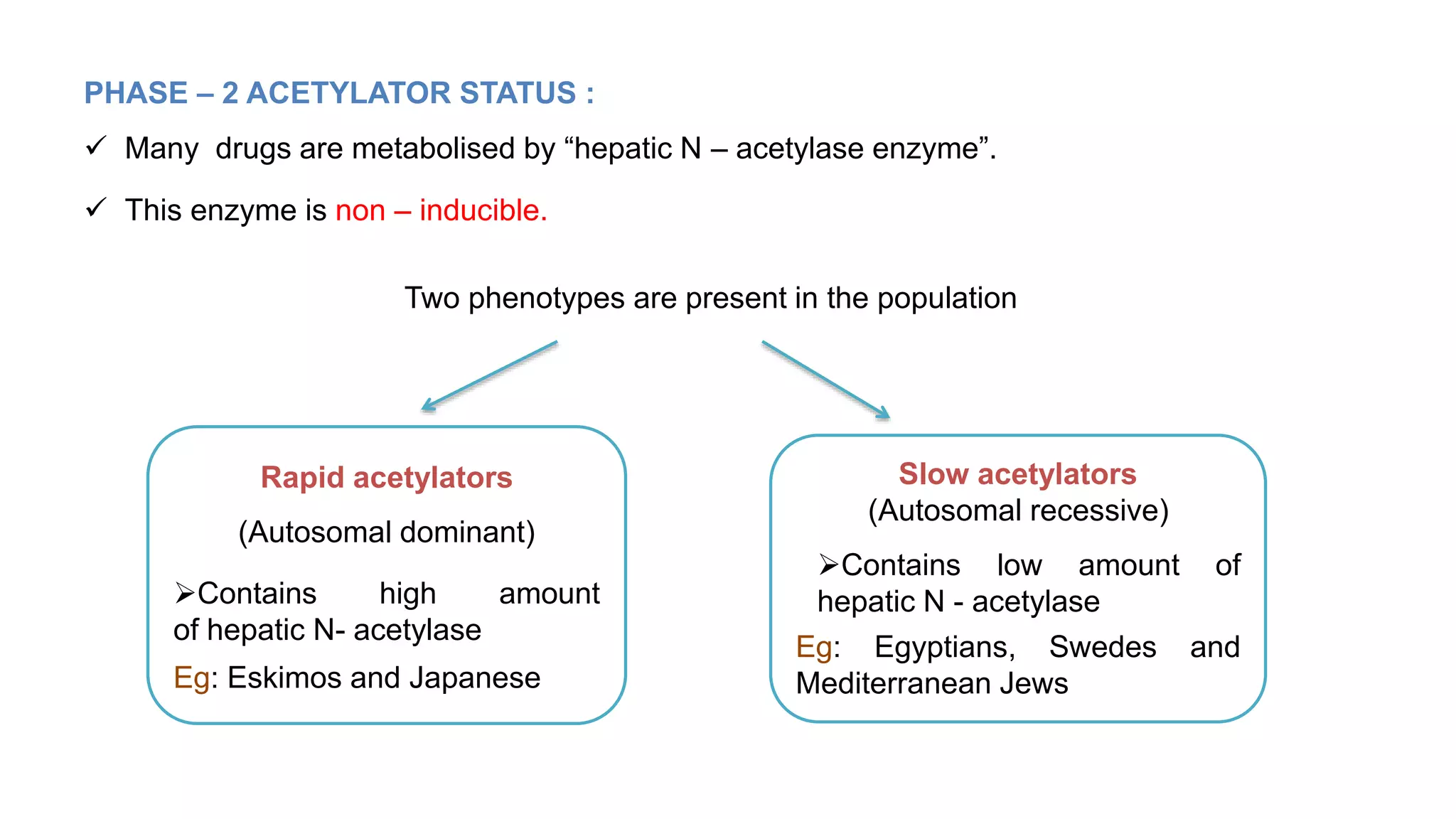
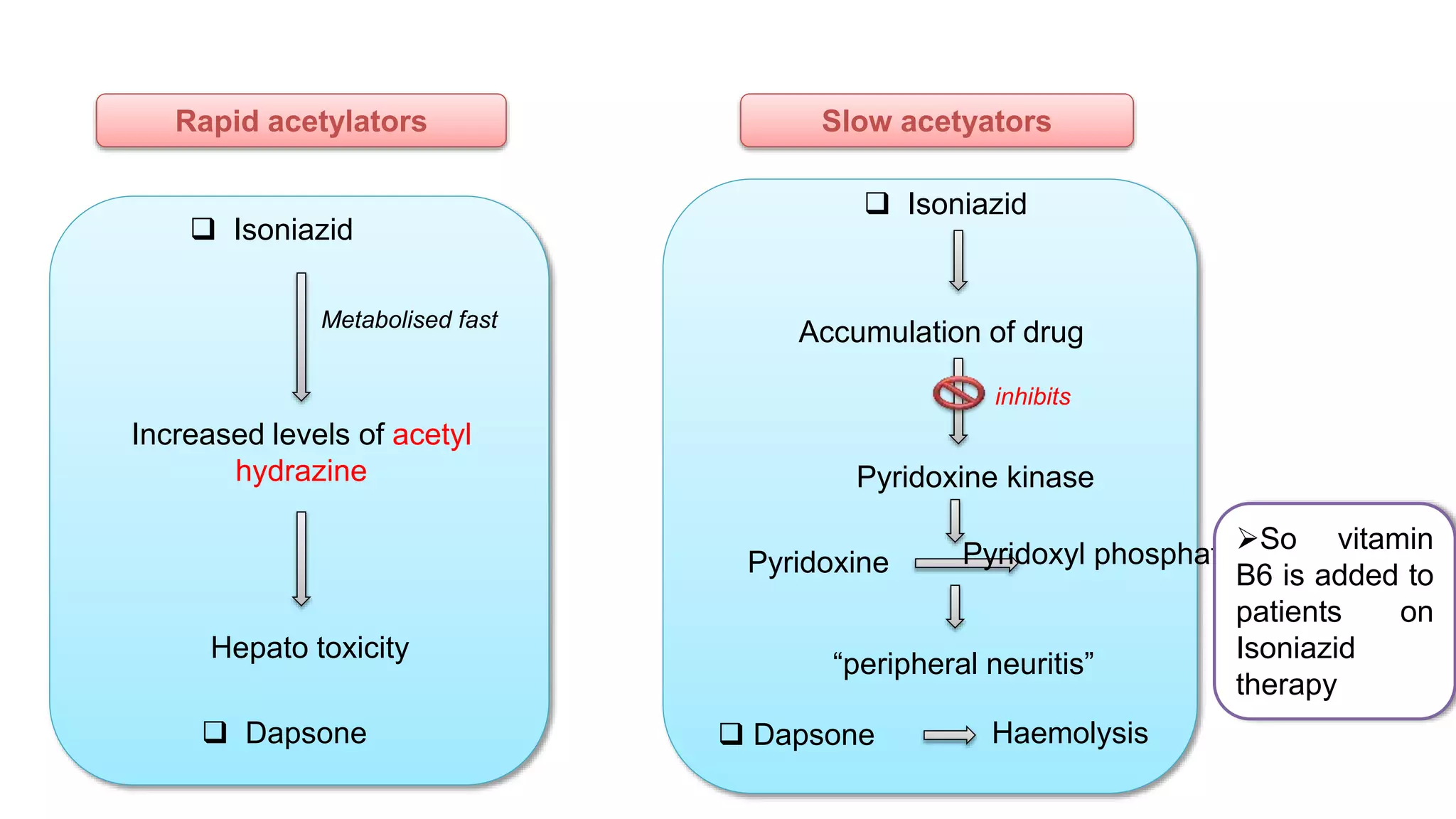
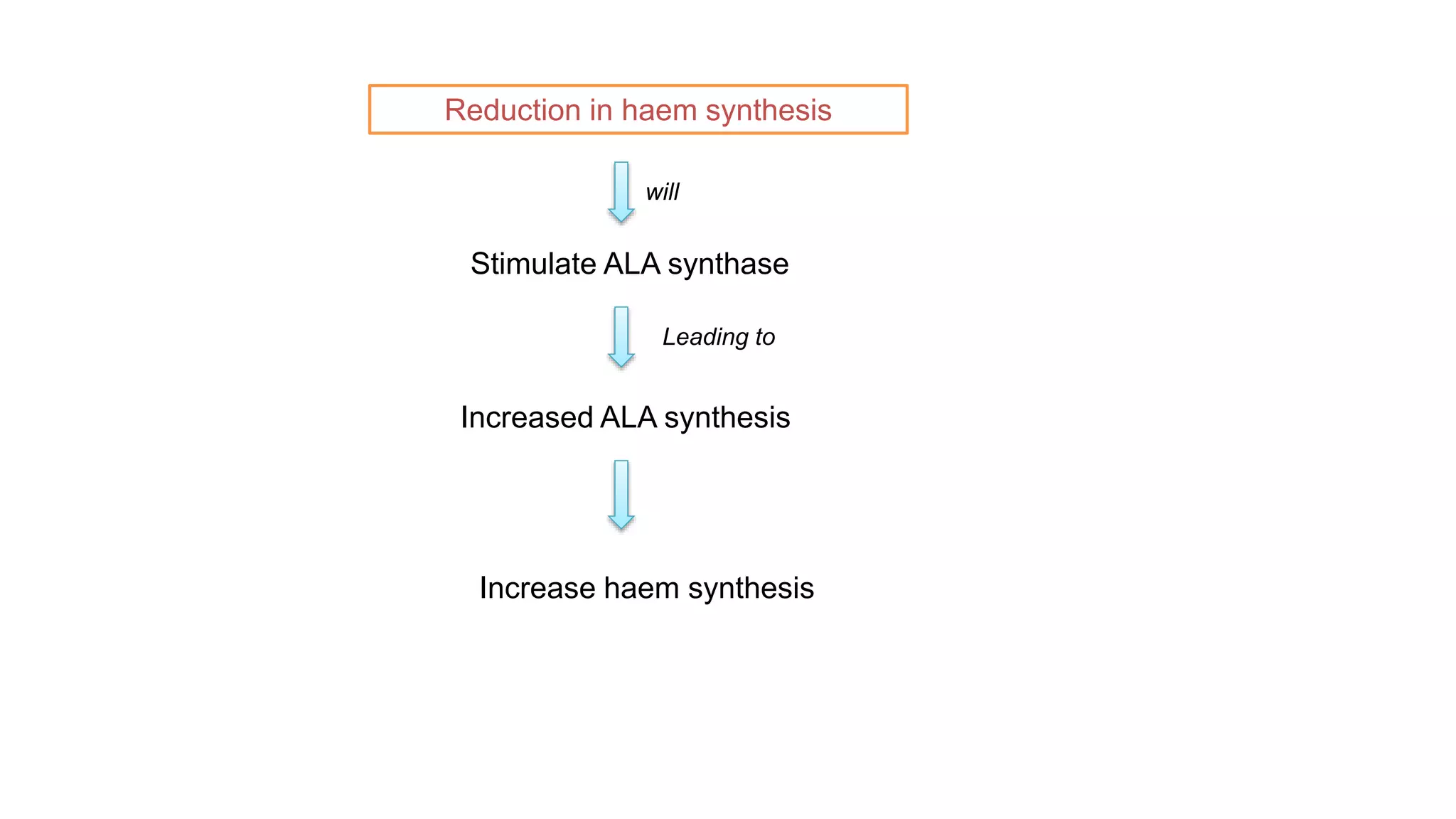
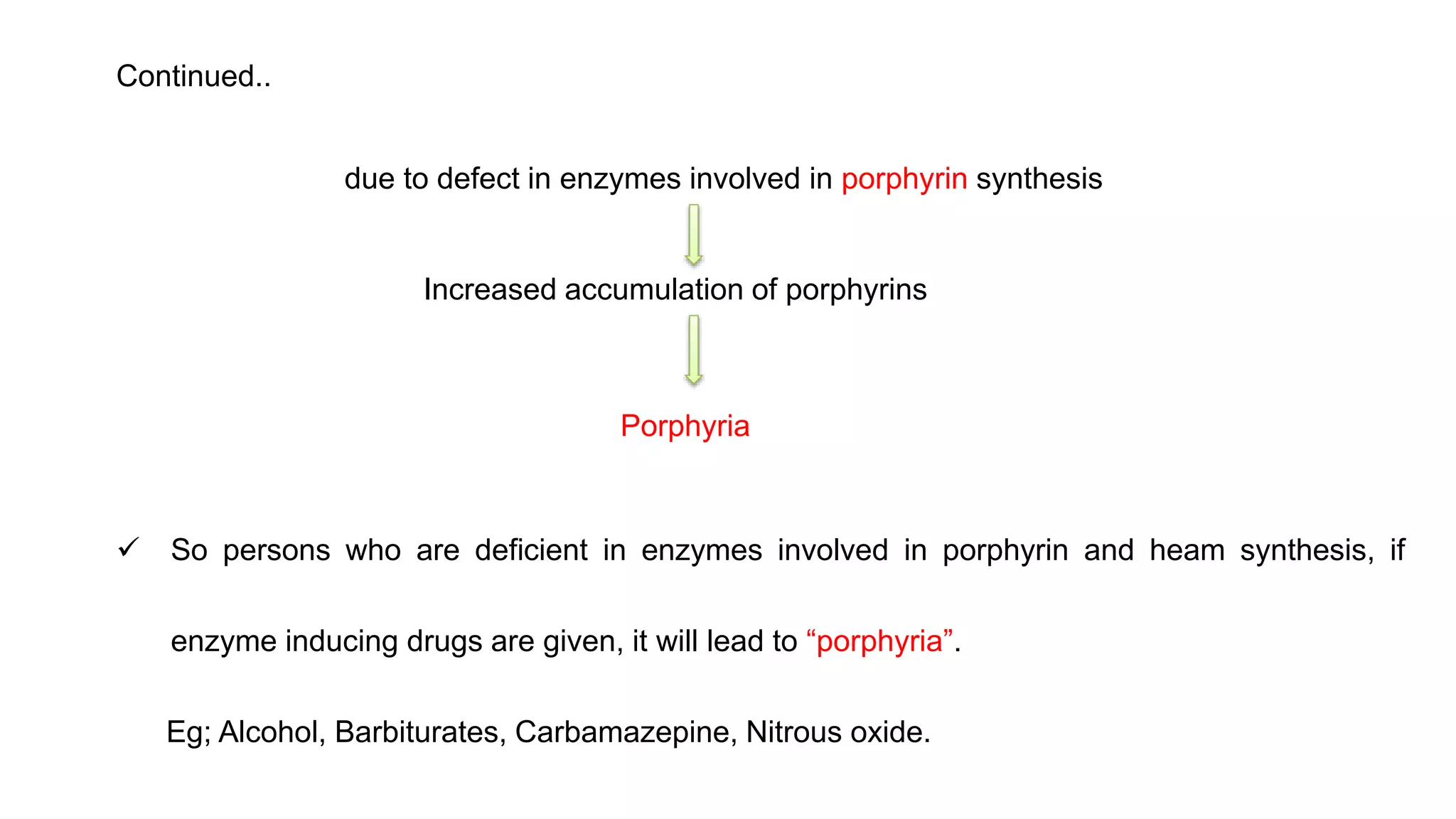
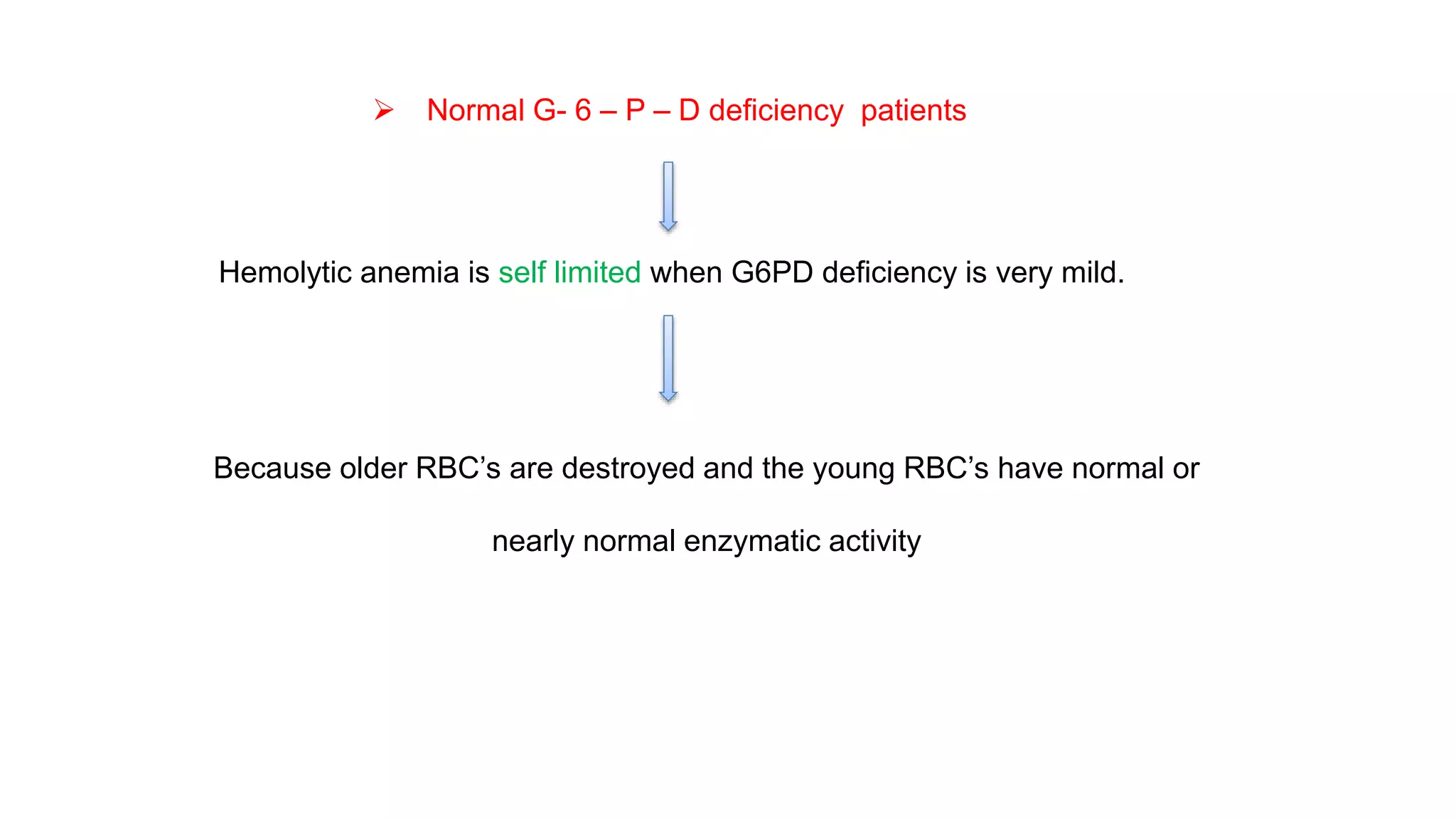
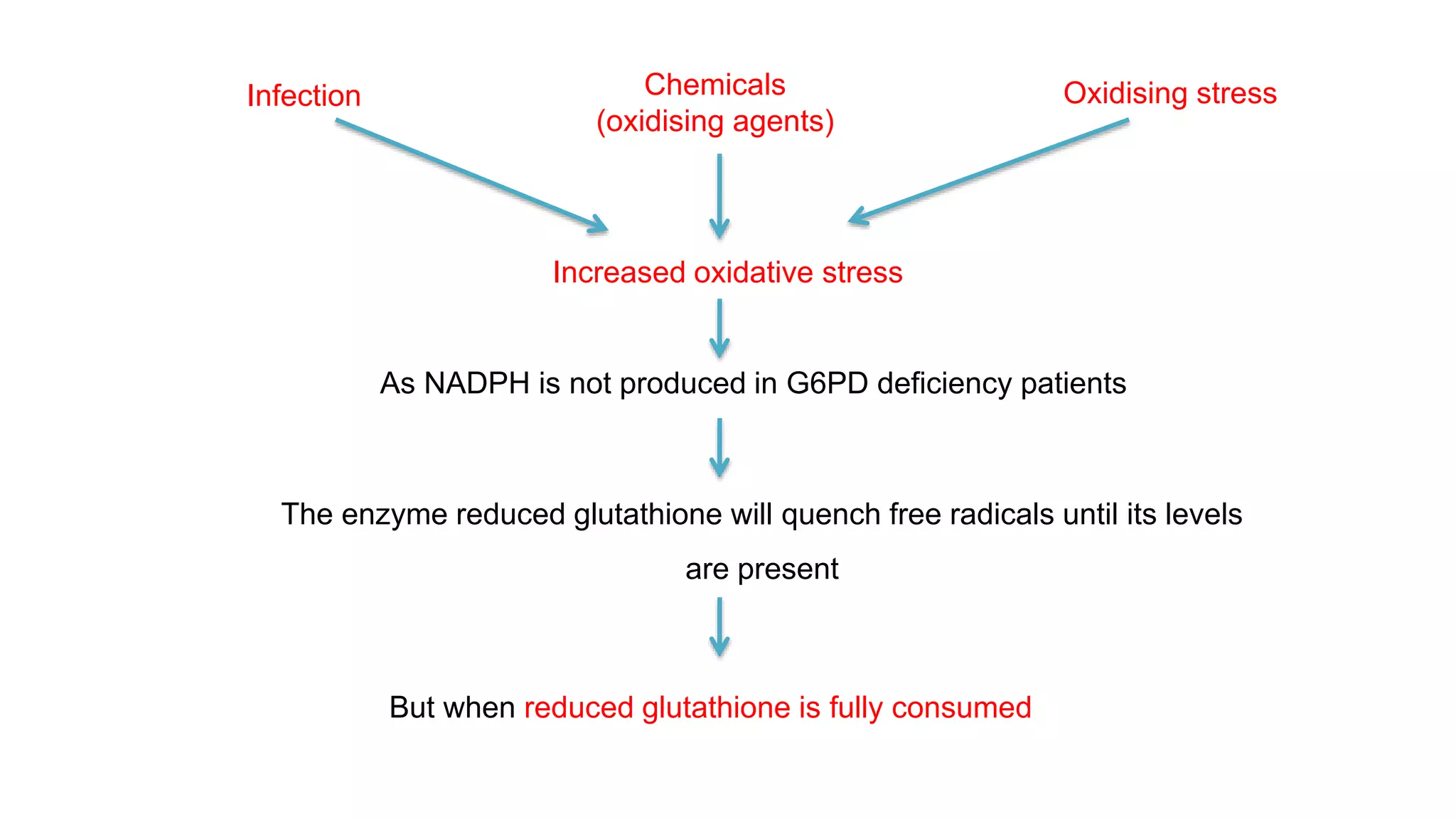
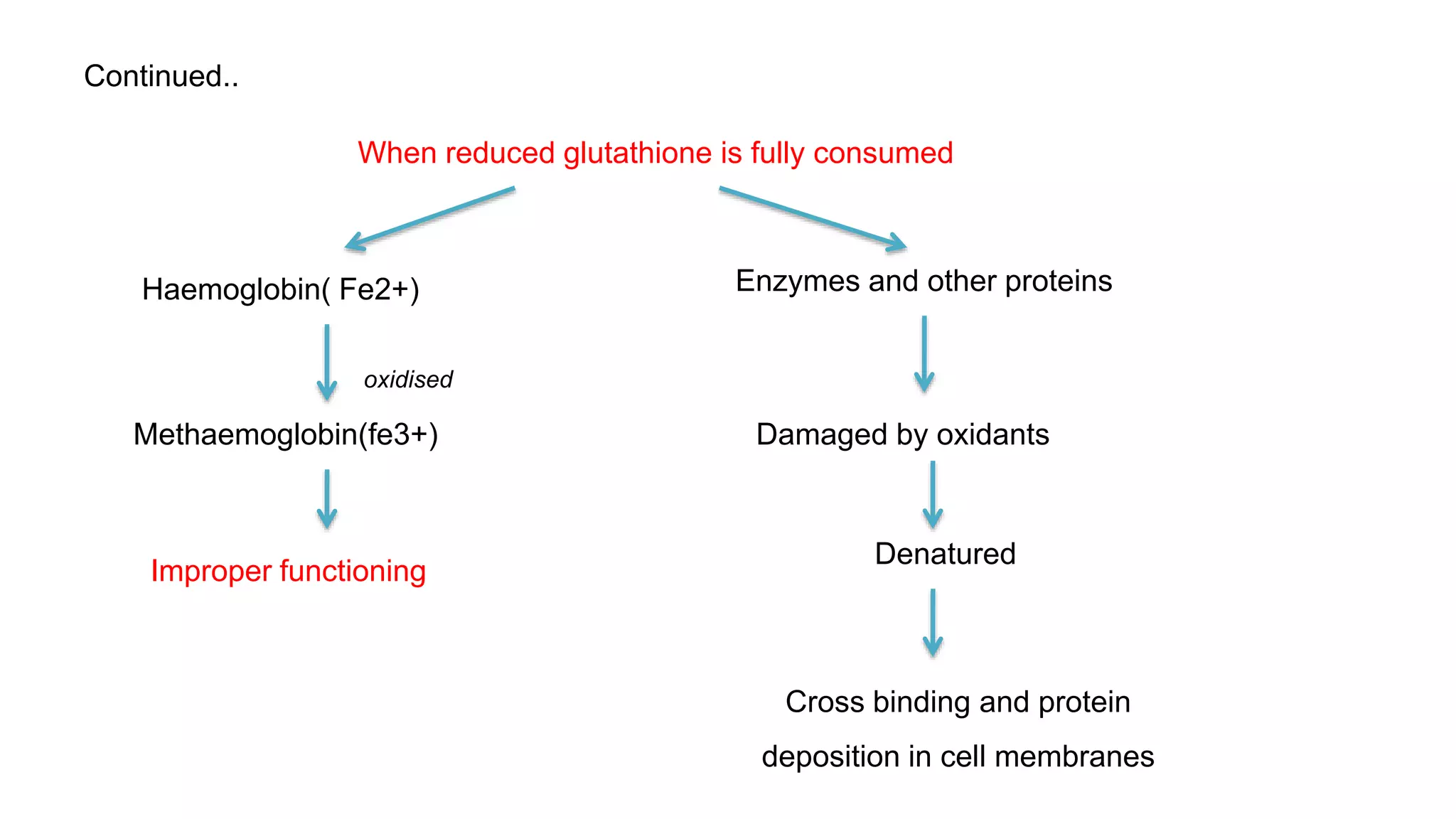
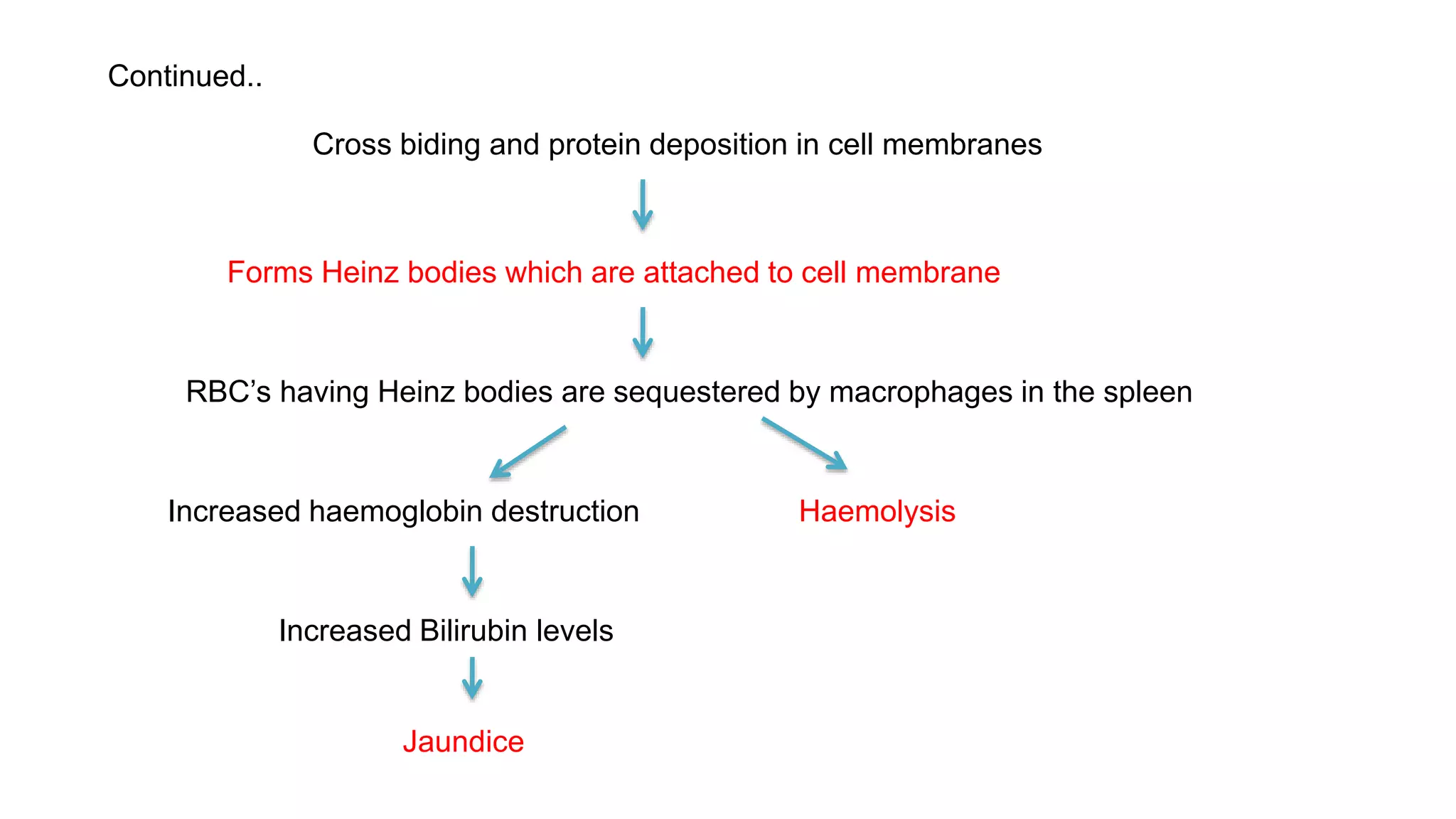
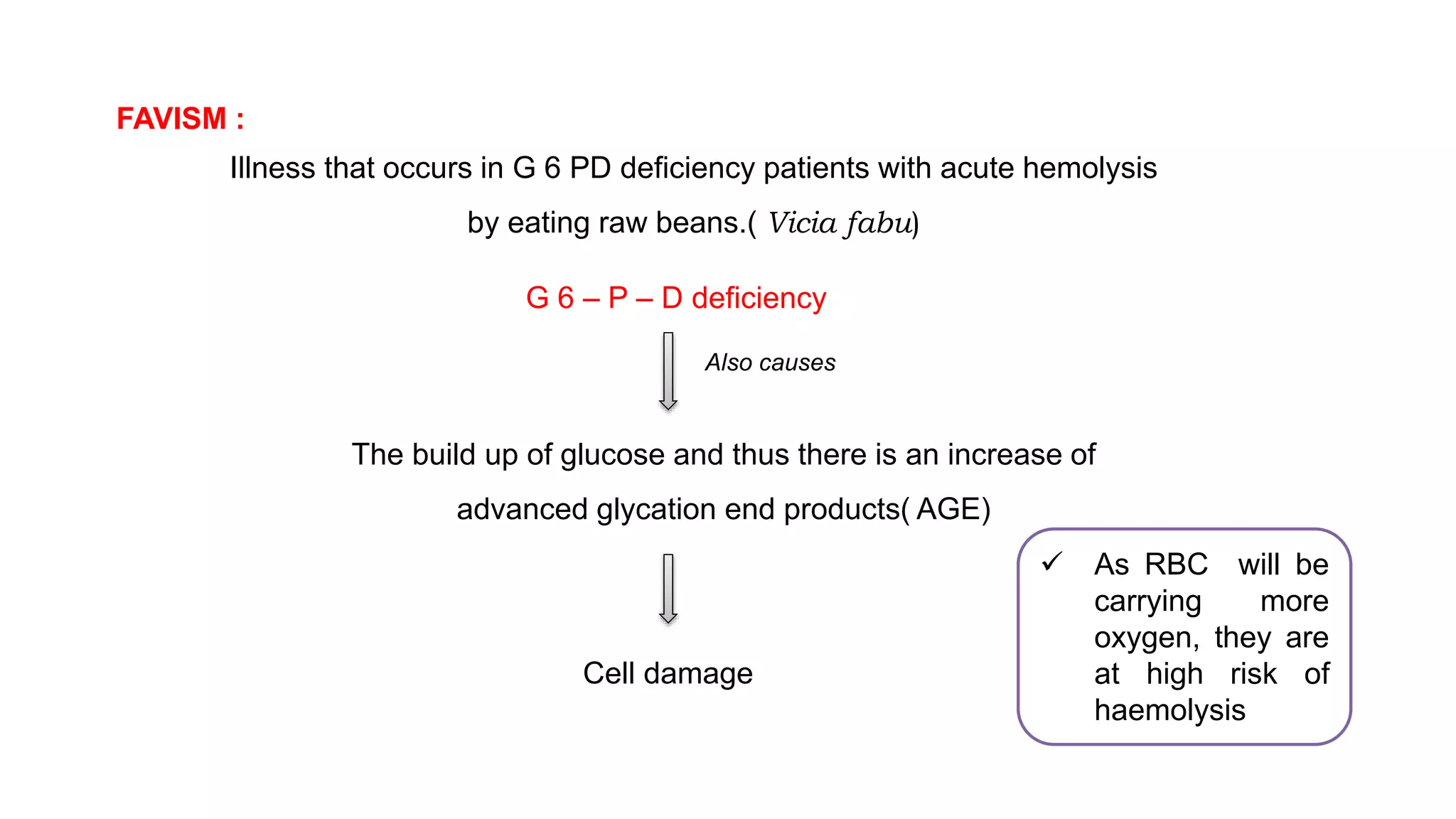
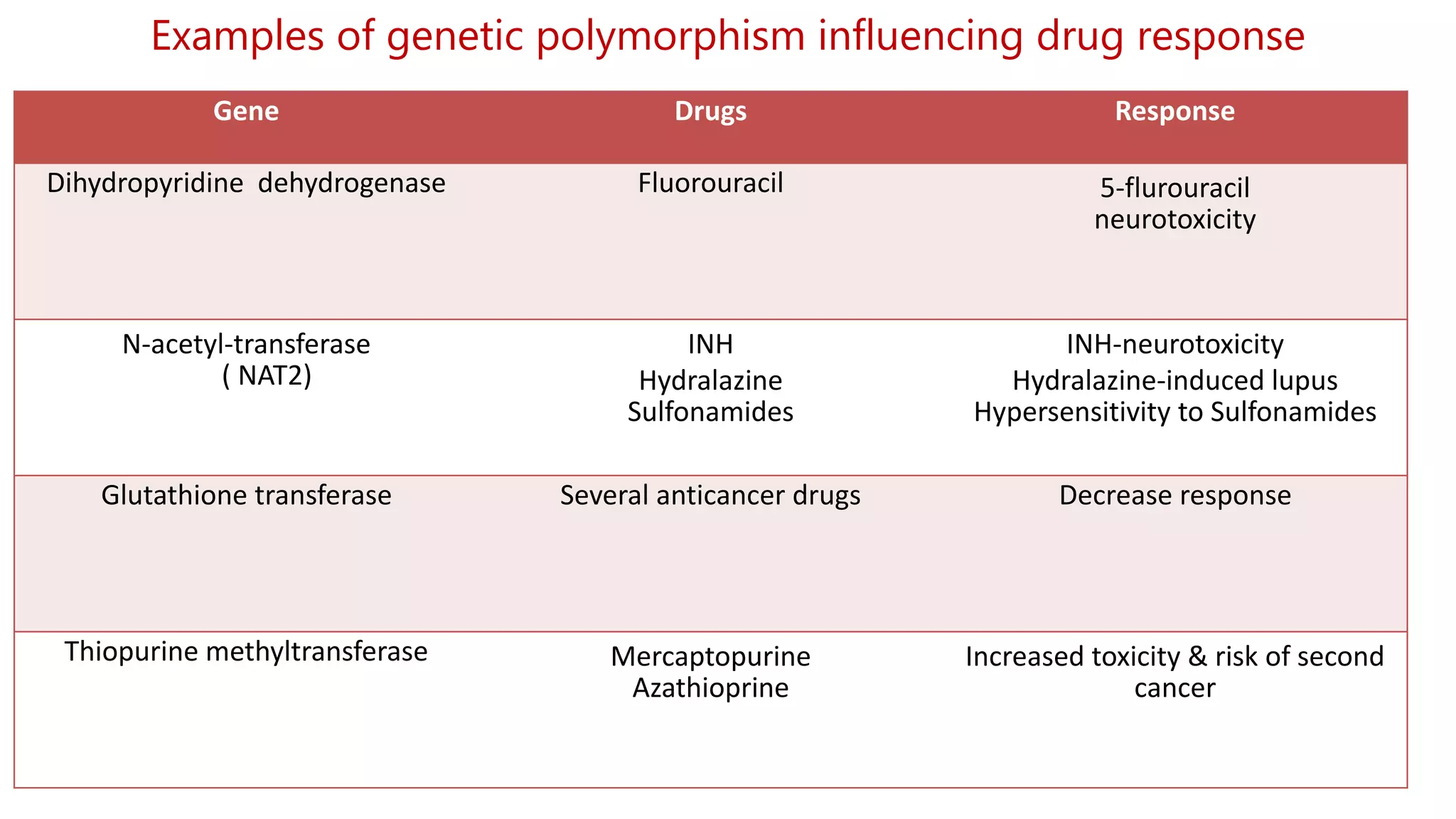
This document discusses pharmacogenetics and individual variation in drug response. It begins by outlining how individuals can metabolize and respond to drugs differently due to genetic factors like polymorphisms. Three key discoveries in the 1950s helped establish the field of pharmacogenetics by showing how genetic differences can impact drug metabolism and efficacy. The document then examines specific genetic variations that influence drug pharmacokinetics and pharmacodynamics, such as differences in metabolizing enzymes and receptors. Understanding these genetic factors can help optimize drug therapy for each individual patient.