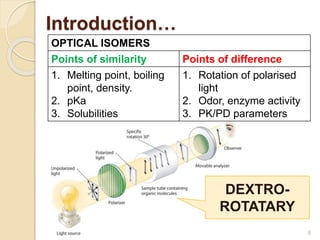
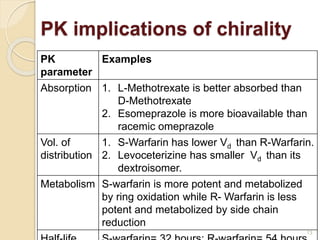
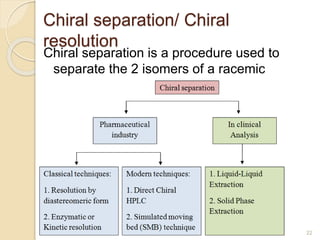
The document discusses the significance of enantiomers, which are chiral compounds that exist in non-superimposable mirror-image forms, and their role in pharmacology. It highlights how most racemic drugs contain a bioactive enantiomer and the importance of understanding enantiomers in terms of drug efficacy and safety, as demonstrated by historical instances like the thalidomide tragedy. It emphasizes current regulatory considerations and the need for chiral separation in the development of new drugs.