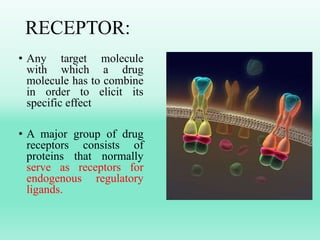
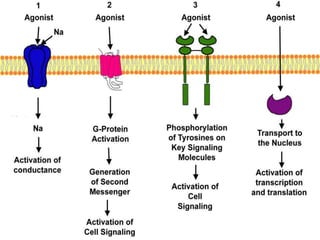
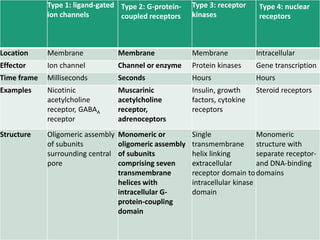
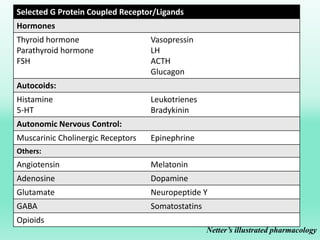
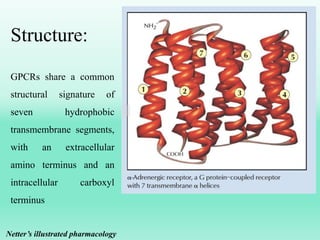
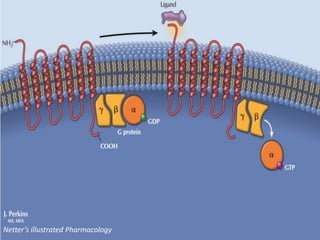
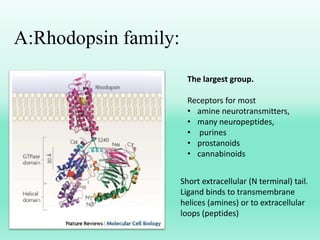
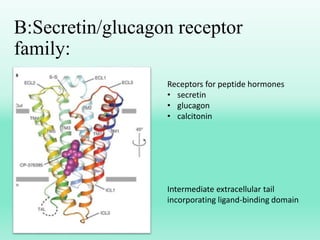
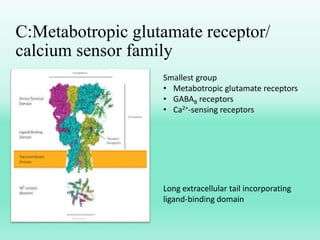
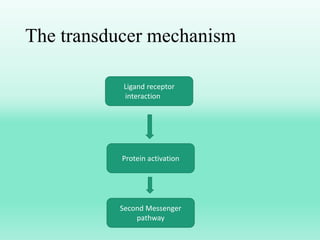
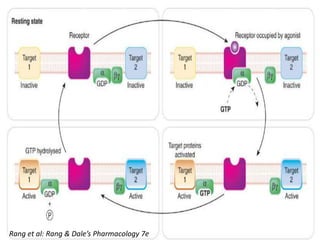
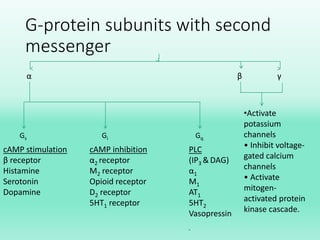
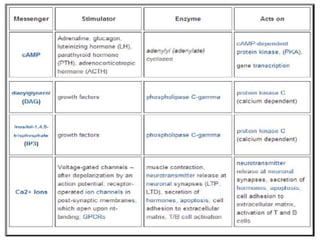
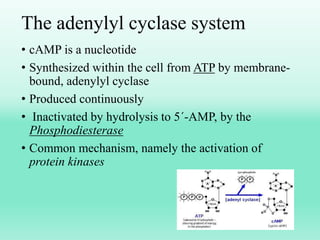
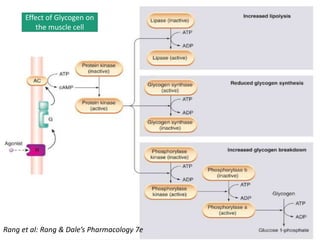
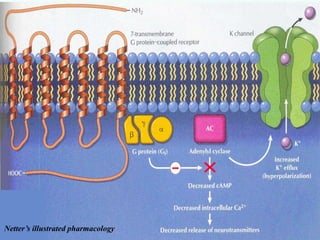
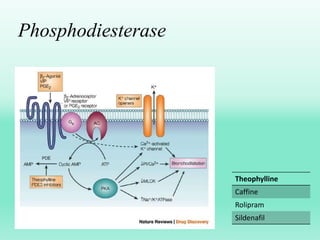
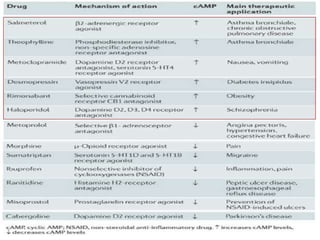
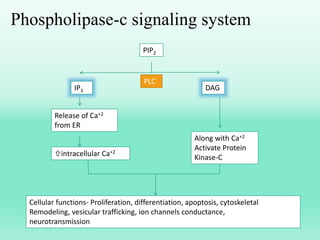
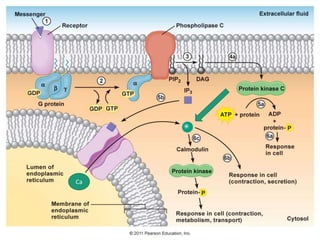
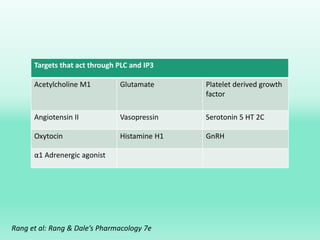
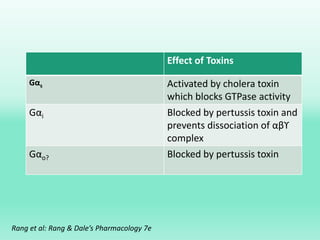
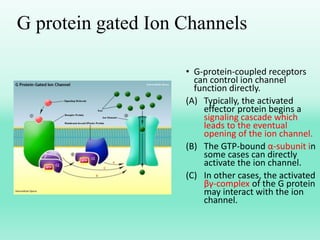
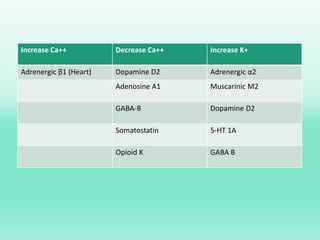
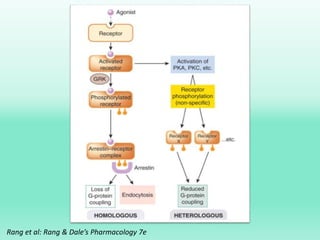
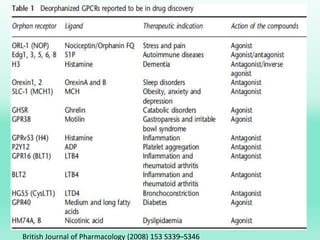
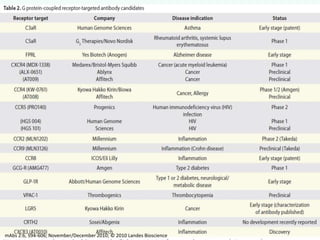
G-protein coupled receptors (GPCRs) are the largest family of membrane receptors and the target of many drugs. They have seven transmembrane domains and signal through G proteins and second messengers like cAMP or IP3. Upon ligand binding, the GPCR activates a G protein that then activates or inhibits downstream effectors to produce a cellular response. GPCRs regulate many physiological functions and half of all drugs target these receptors. Recent research focuses on deorphaning orphan GPCRs and understanding how GPCR mutations can cause disease.