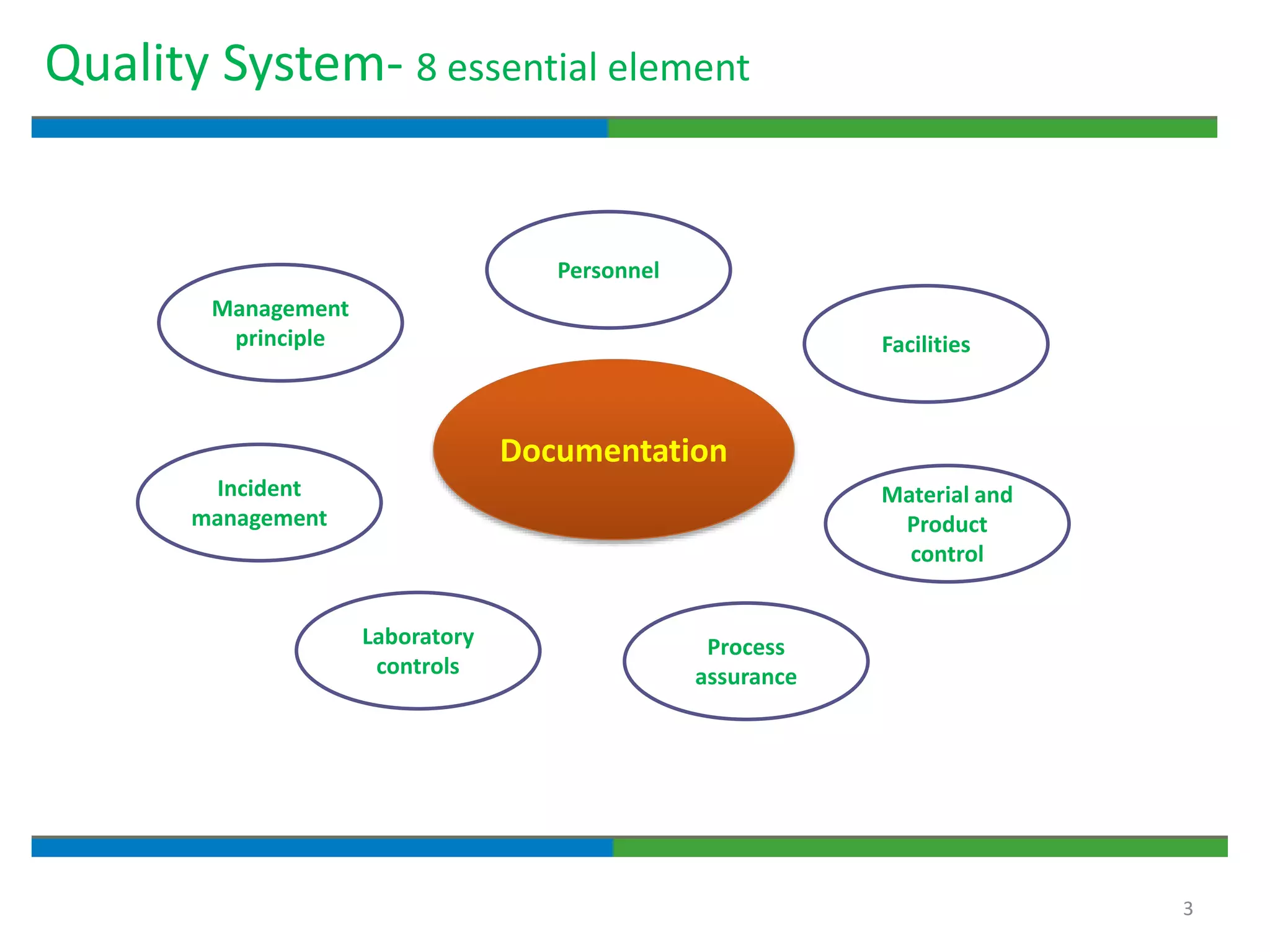
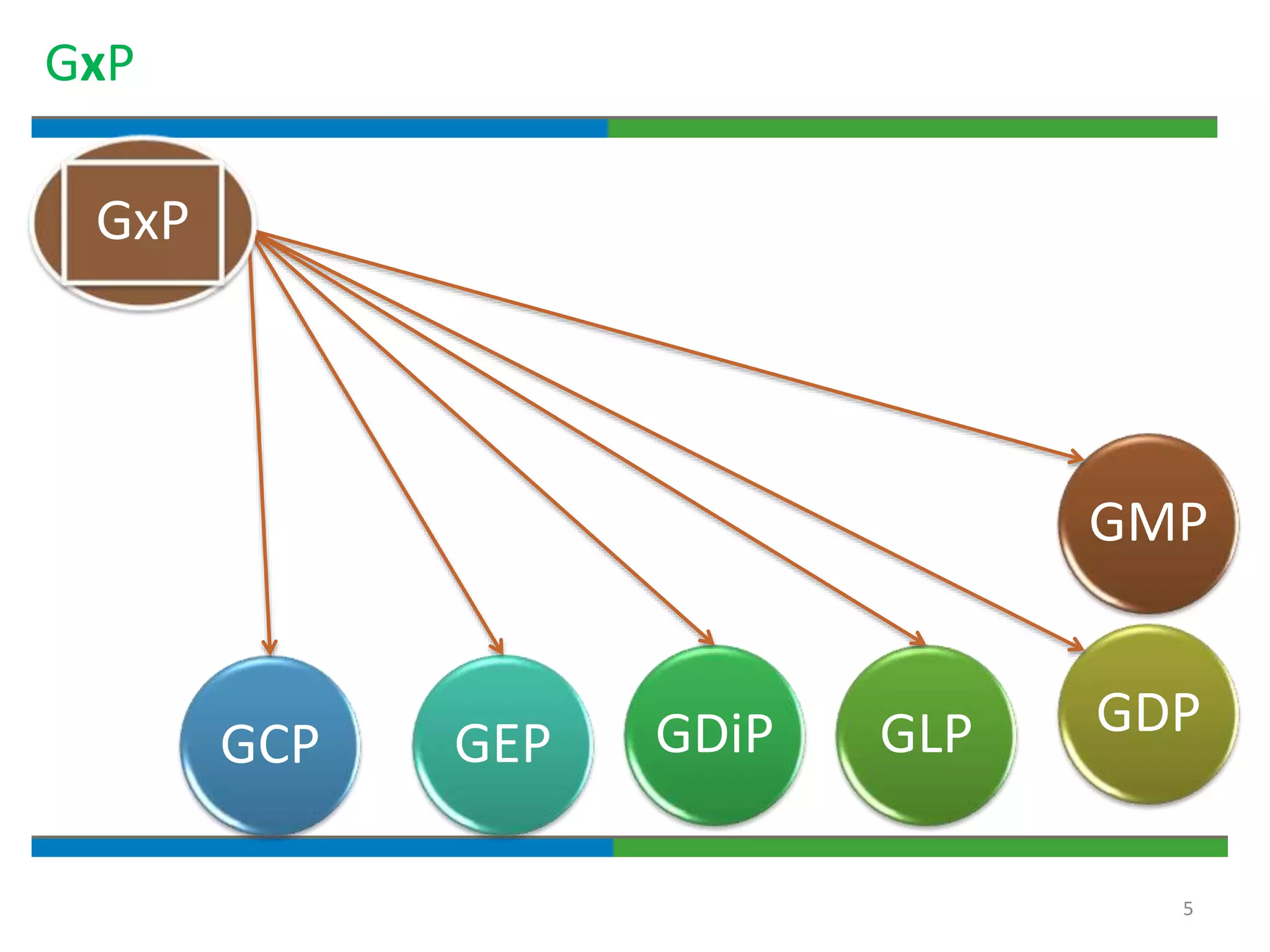
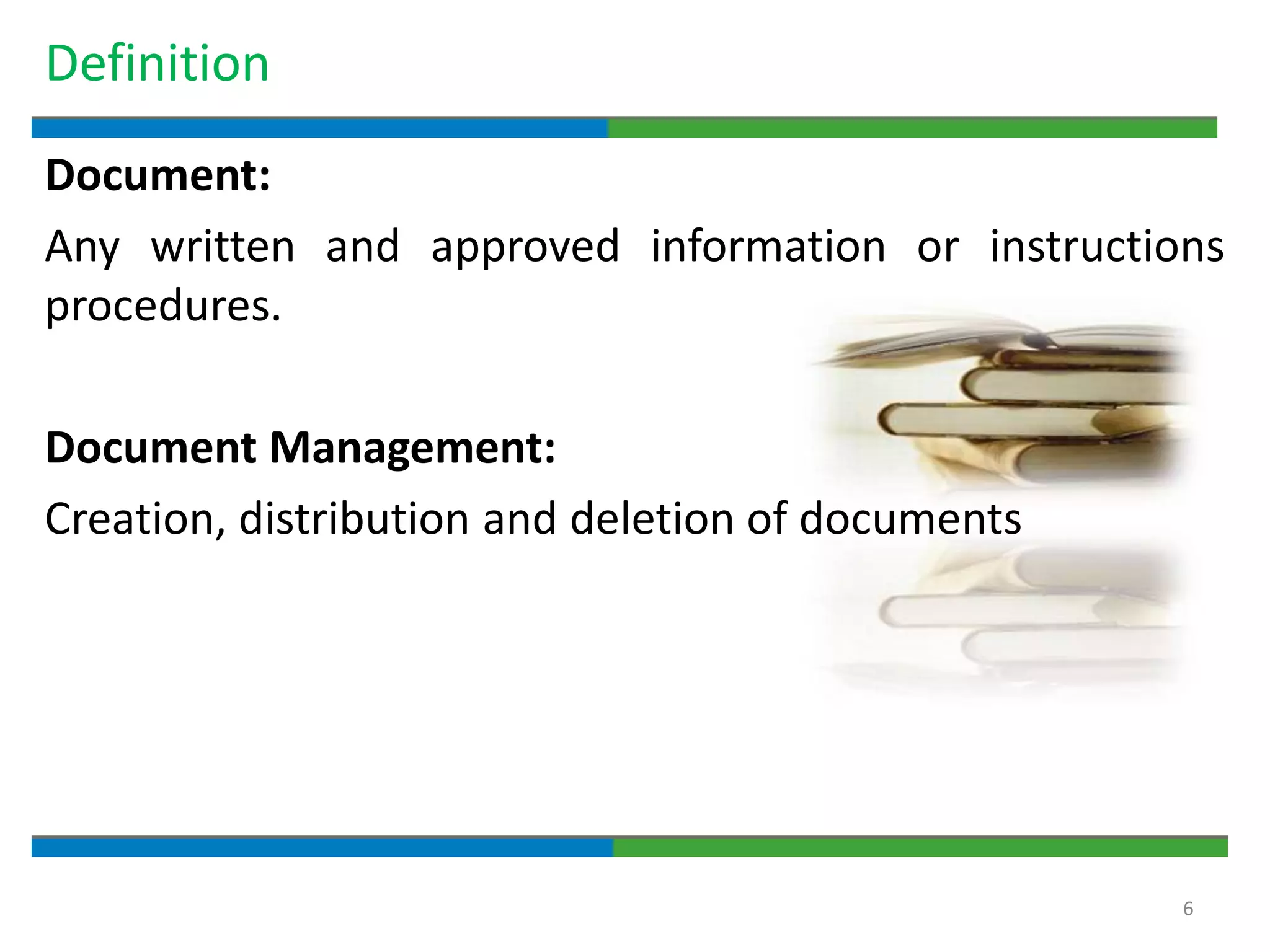
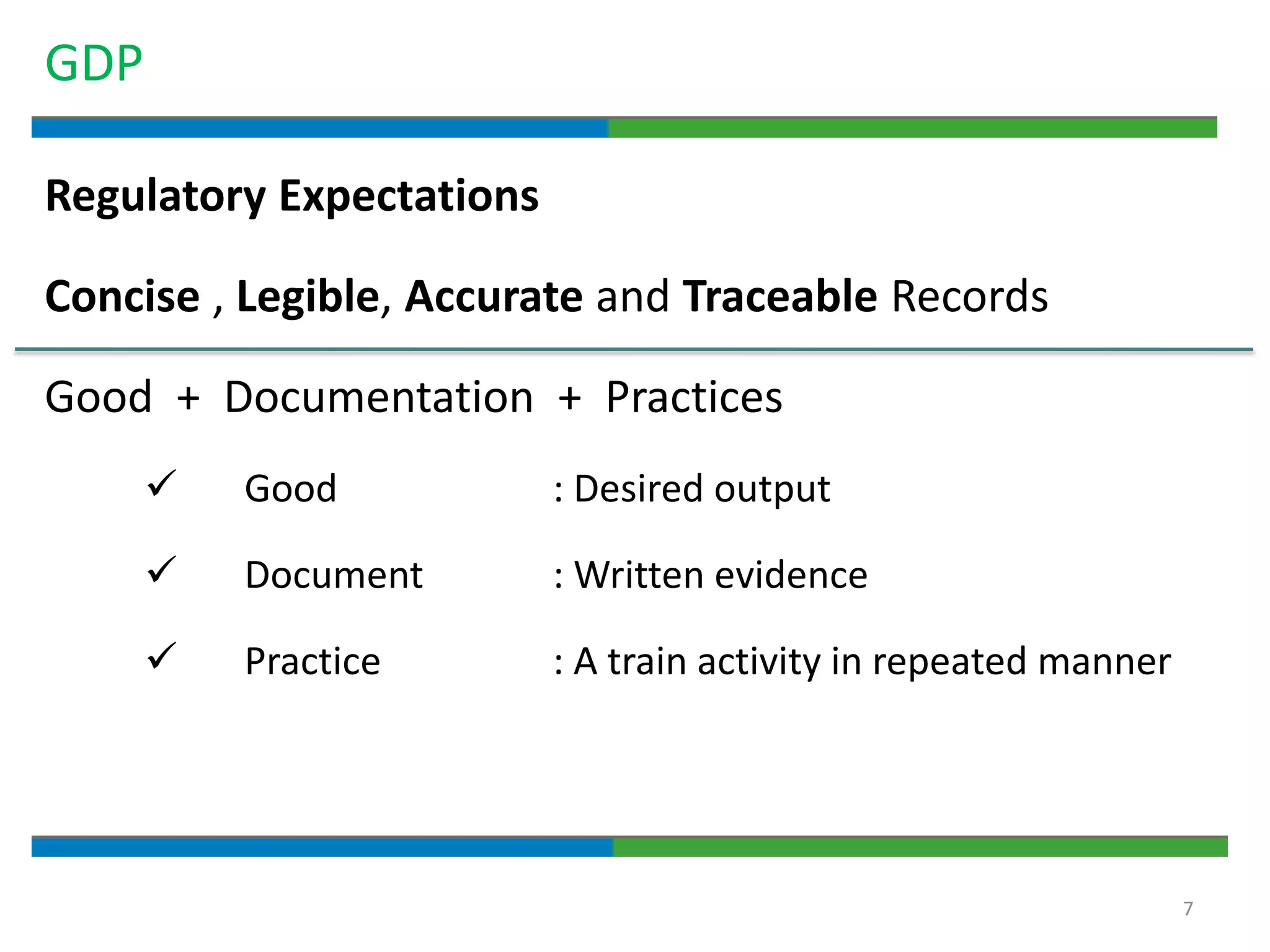
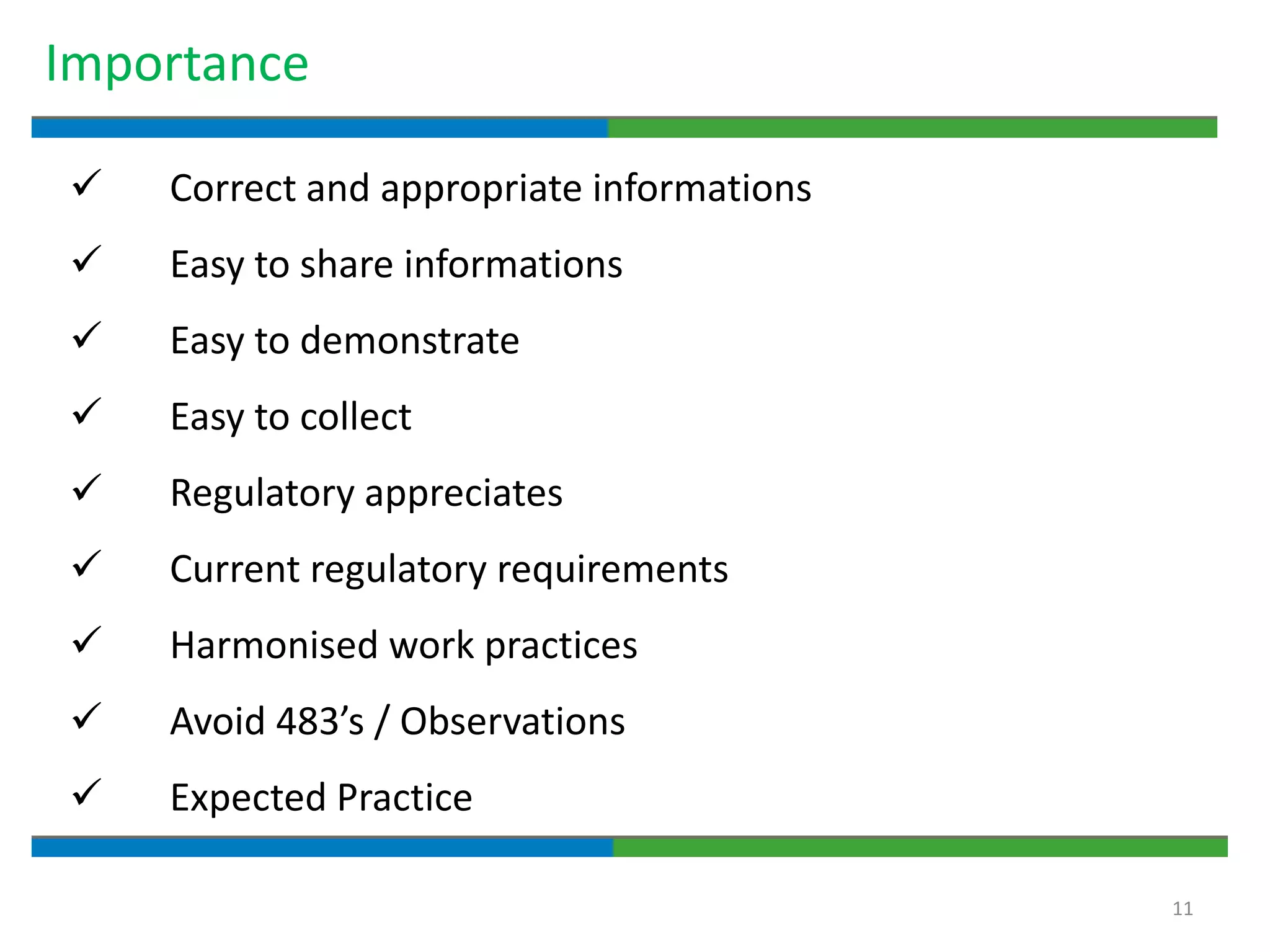
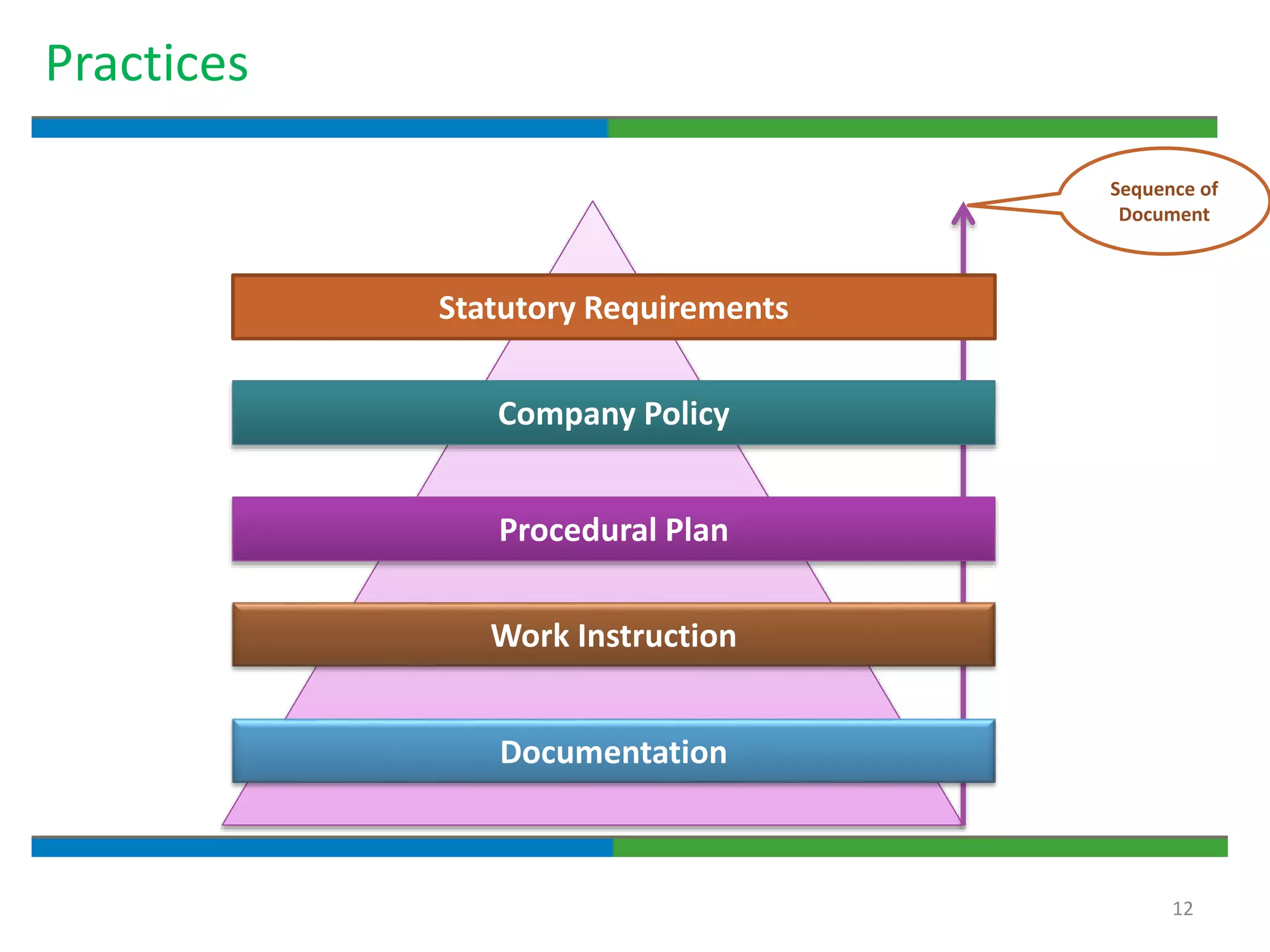
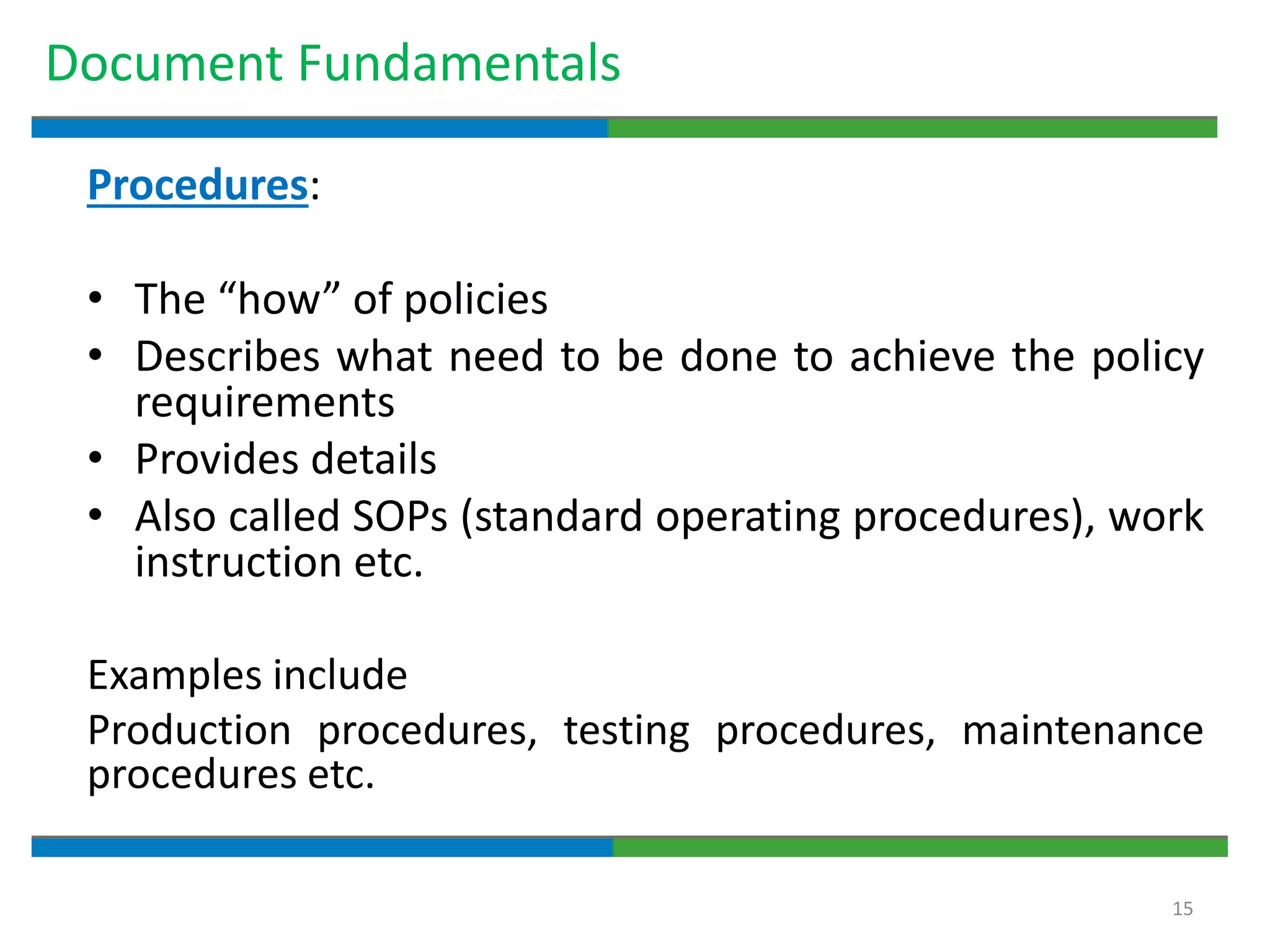
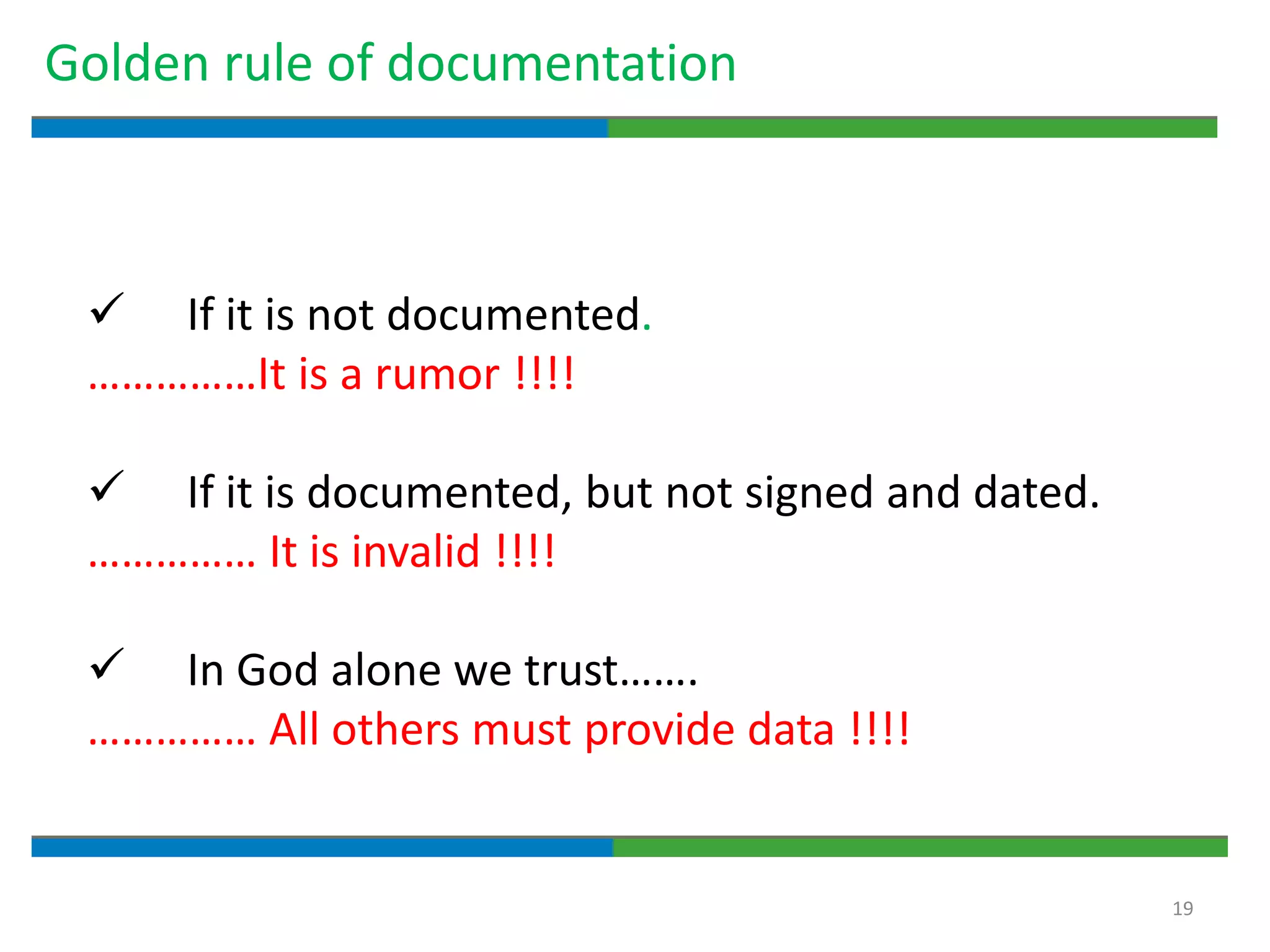
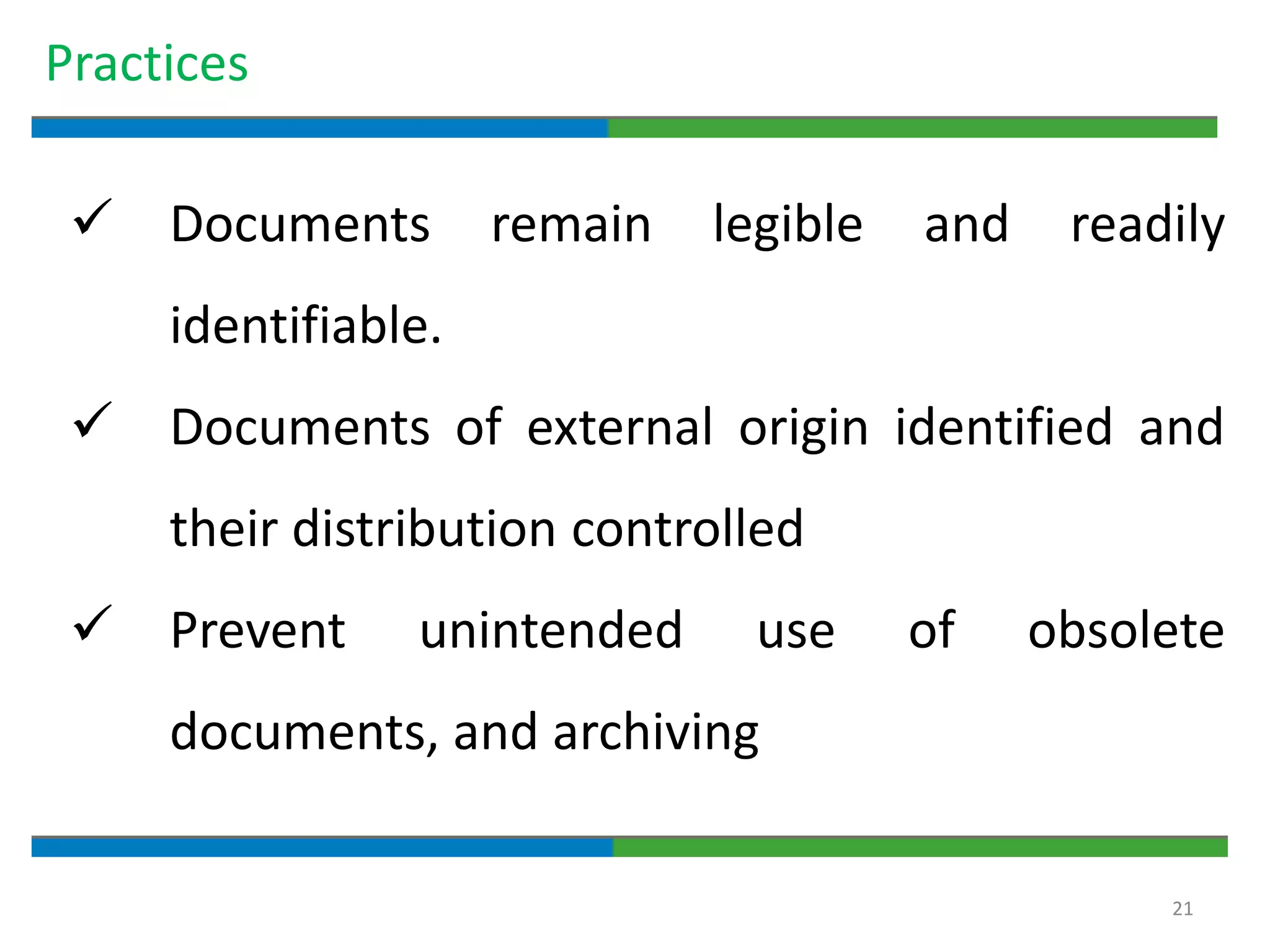
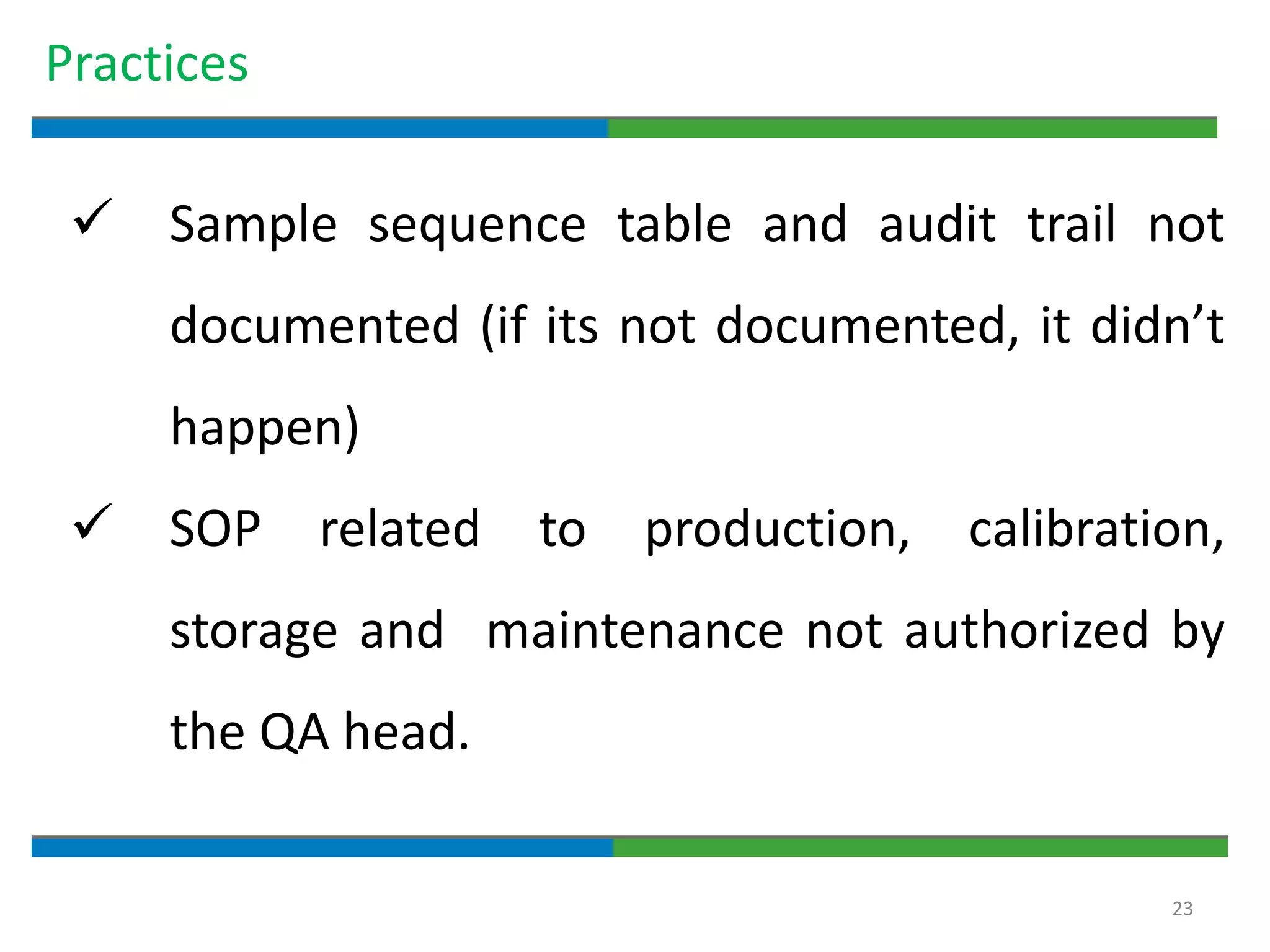
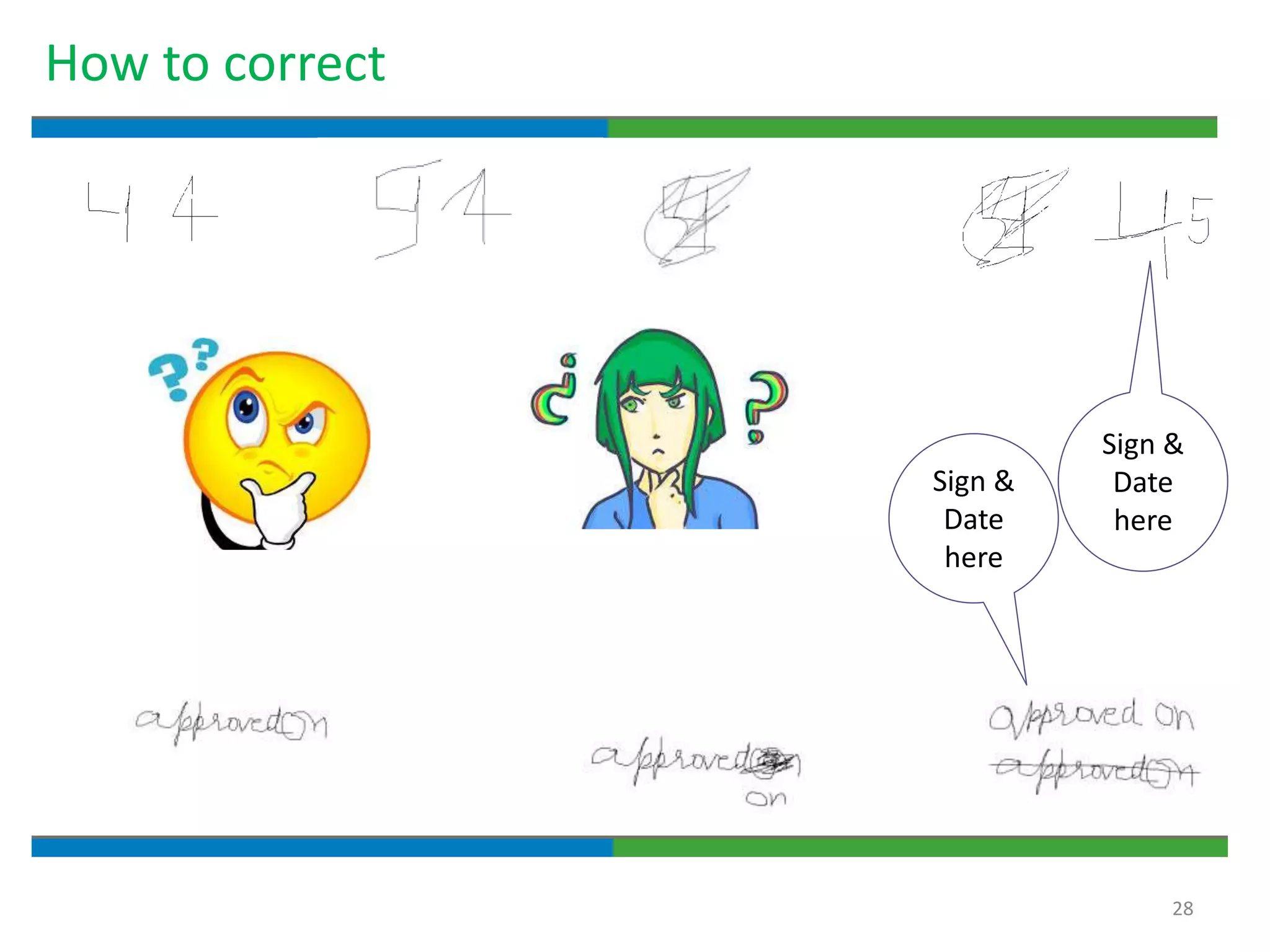
Good documentation practices are essential for quality assurance. The key elements of a quality system include management principles, personnel, documentation, facilities, material and product control, process assurance, and incident management. The "10 Commandments of GMP" establish best practices for writing procedures, following procedures, documenting work, designing facilities, maintaining equipment, validating processes, maintaining cleanliness, ensuring competence, controlling quality, and conducting audits. Proper document practices include generating concise, legible, accurate, and traceable records to demonstrate regulatory compliance and support quality operations.