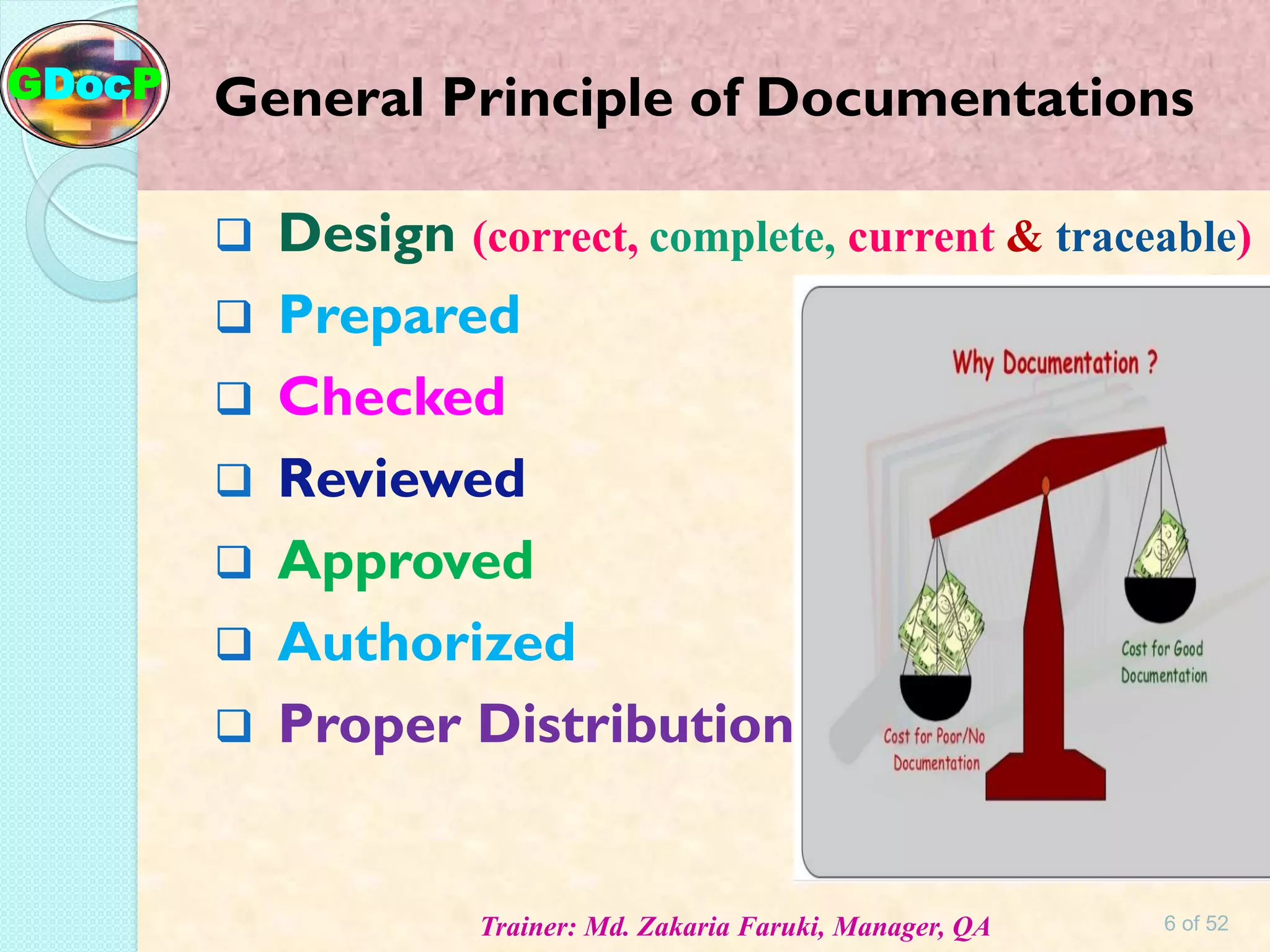
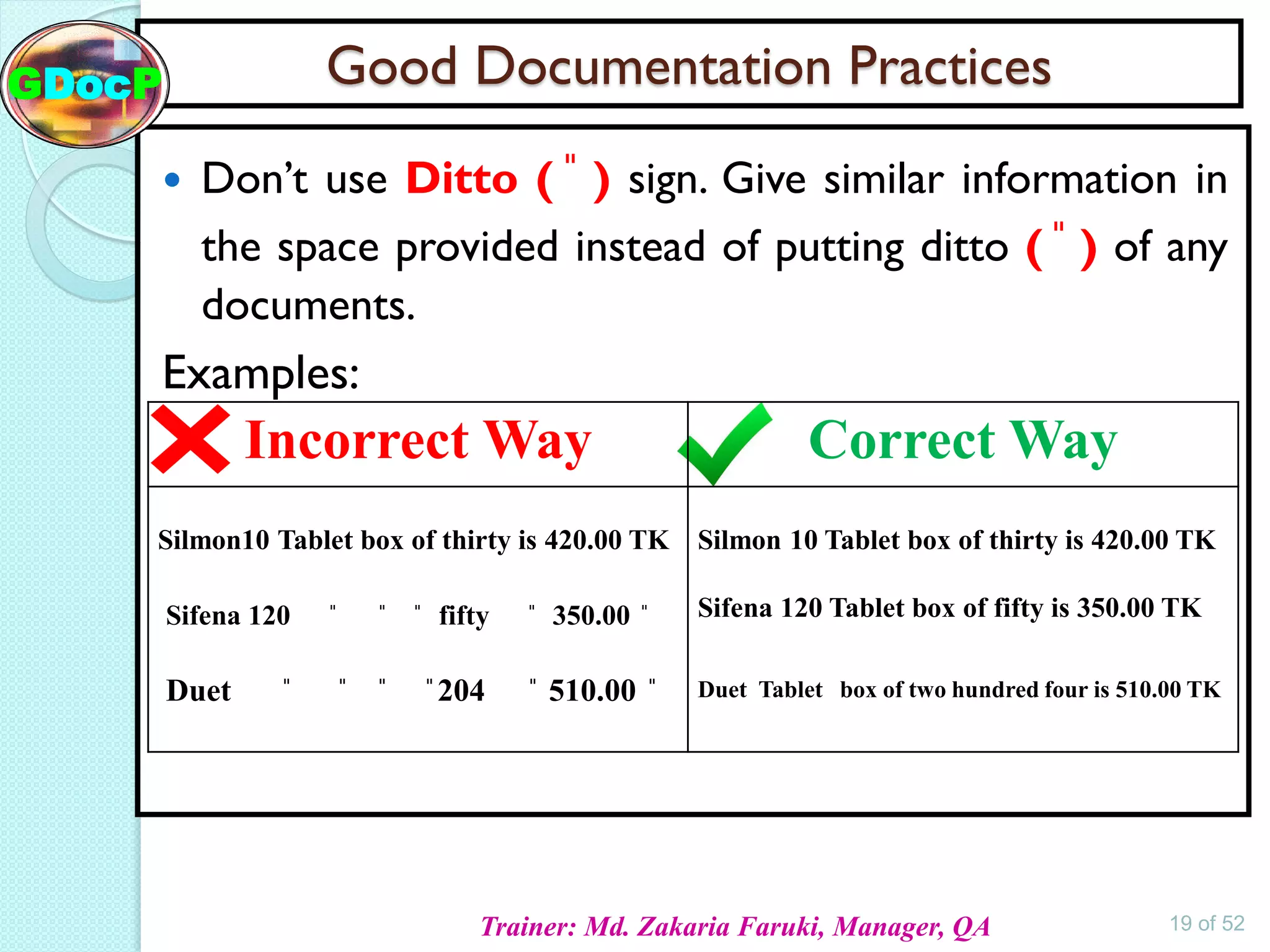
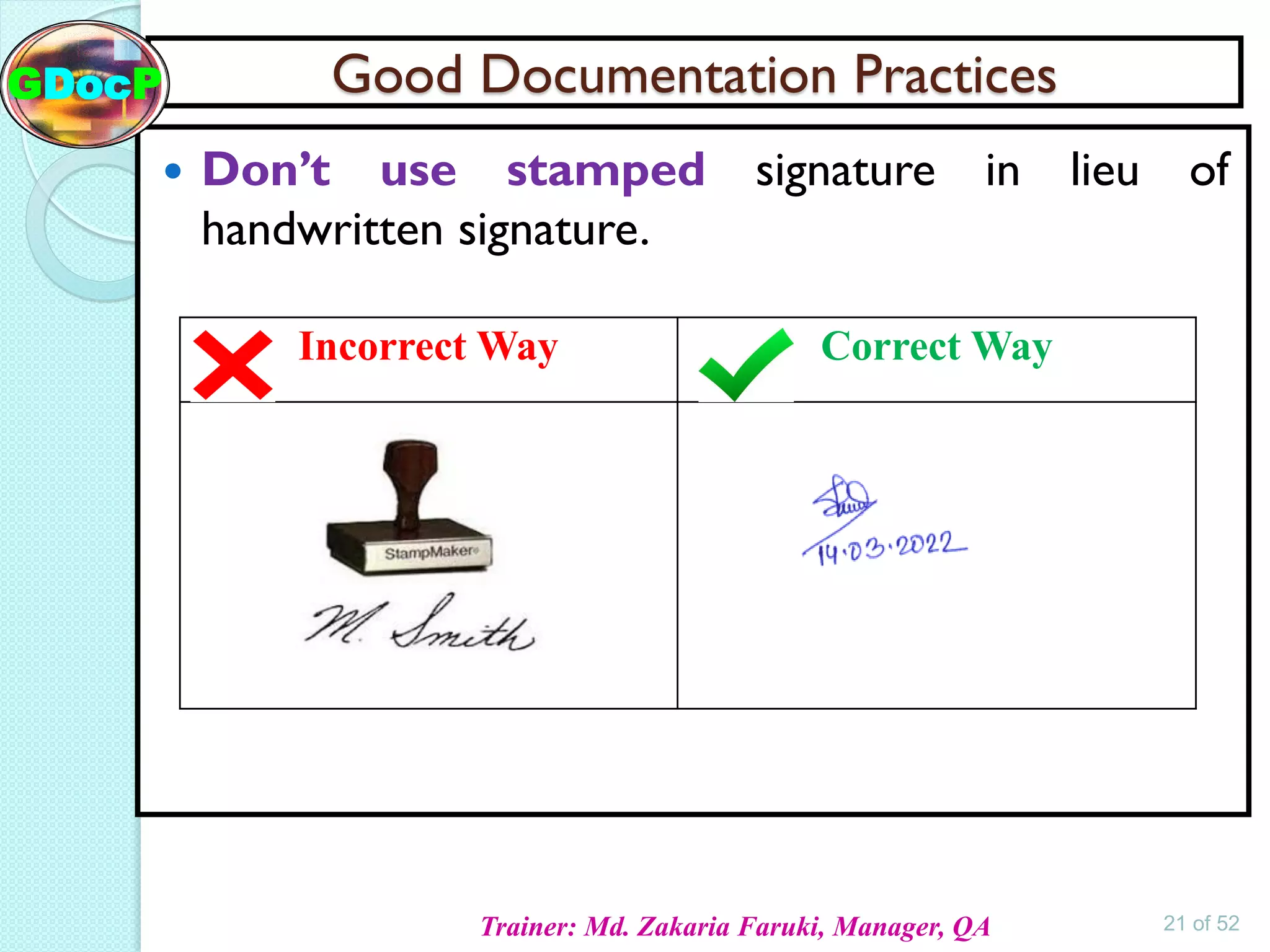
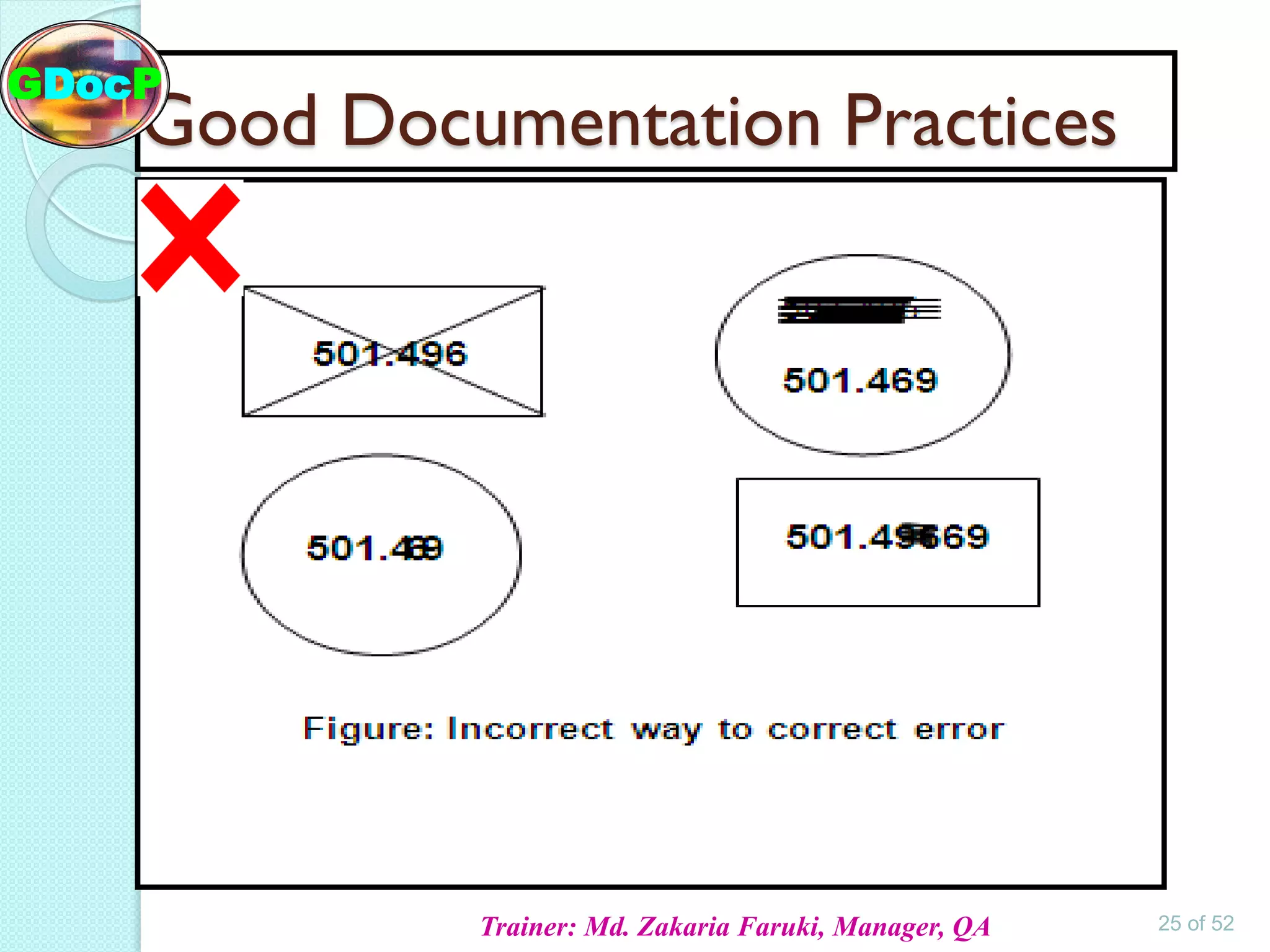
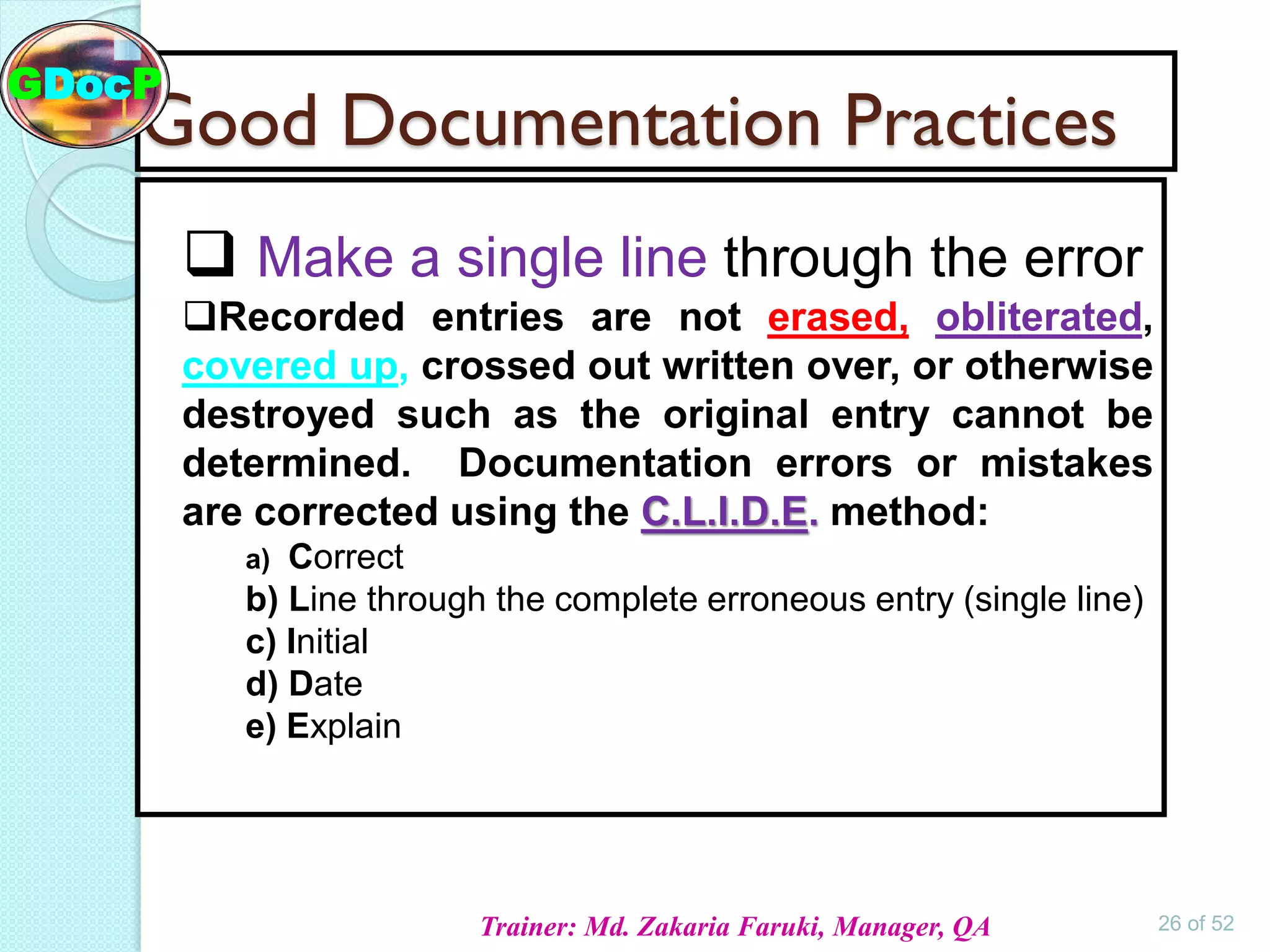
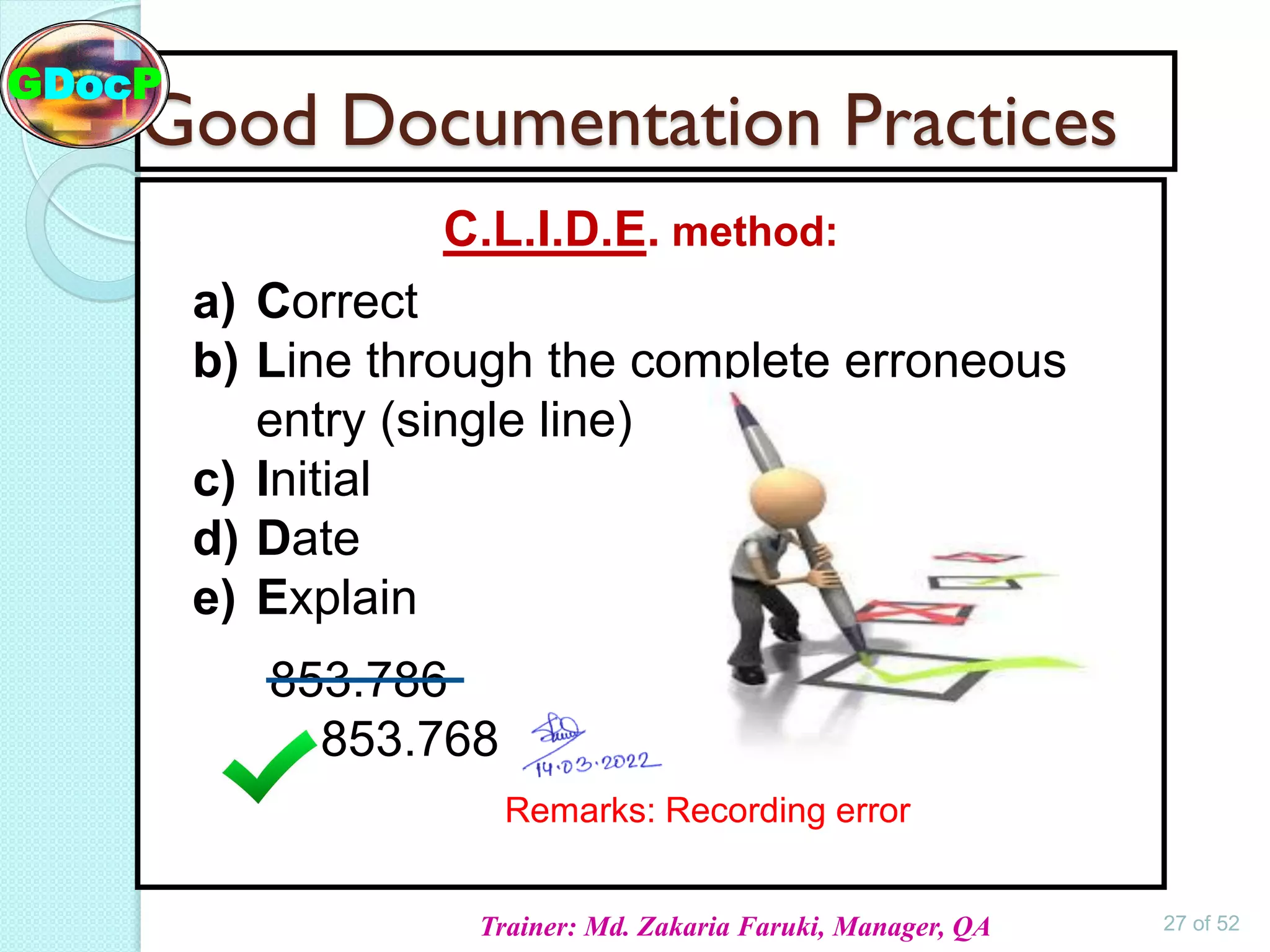
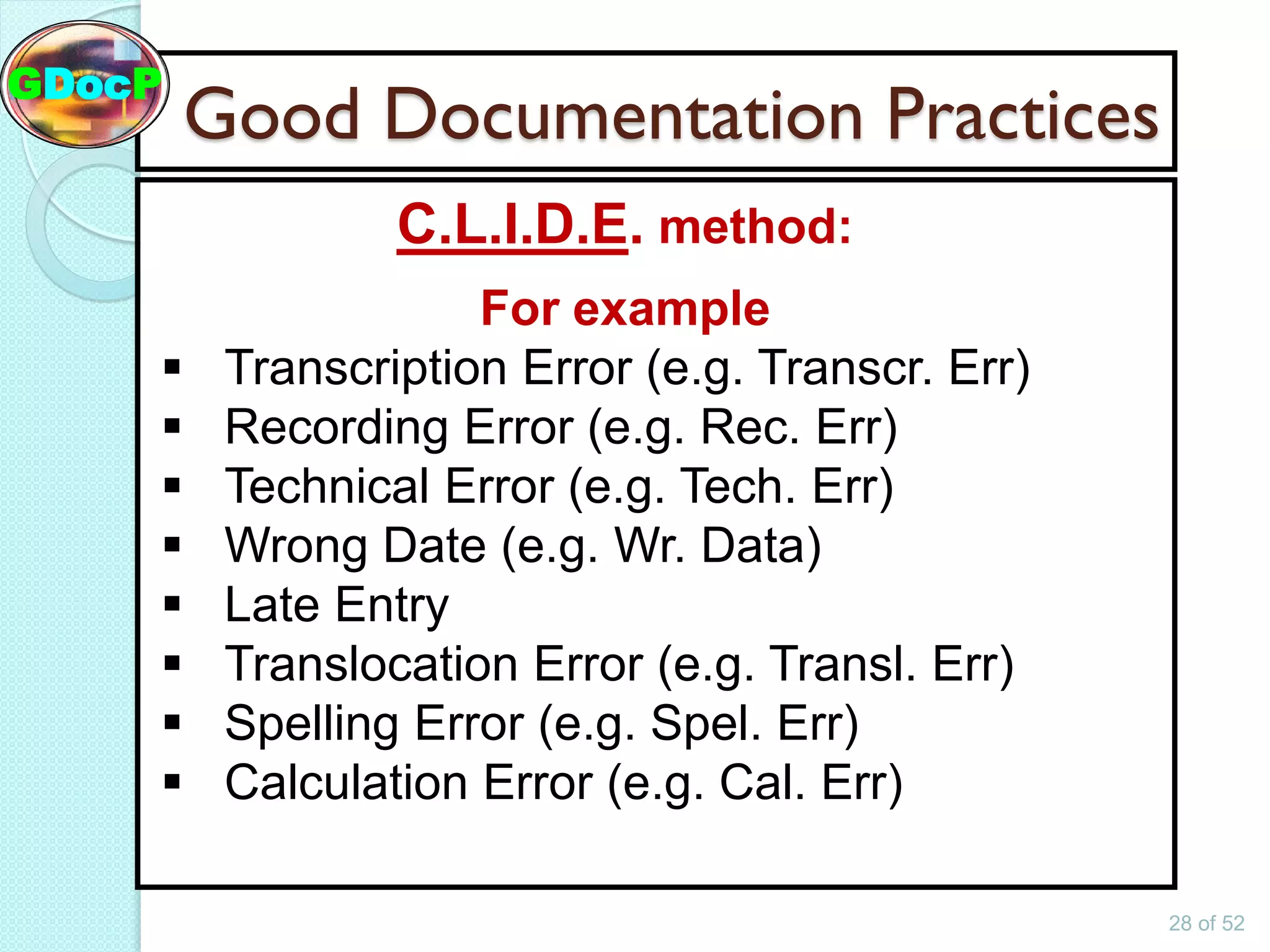
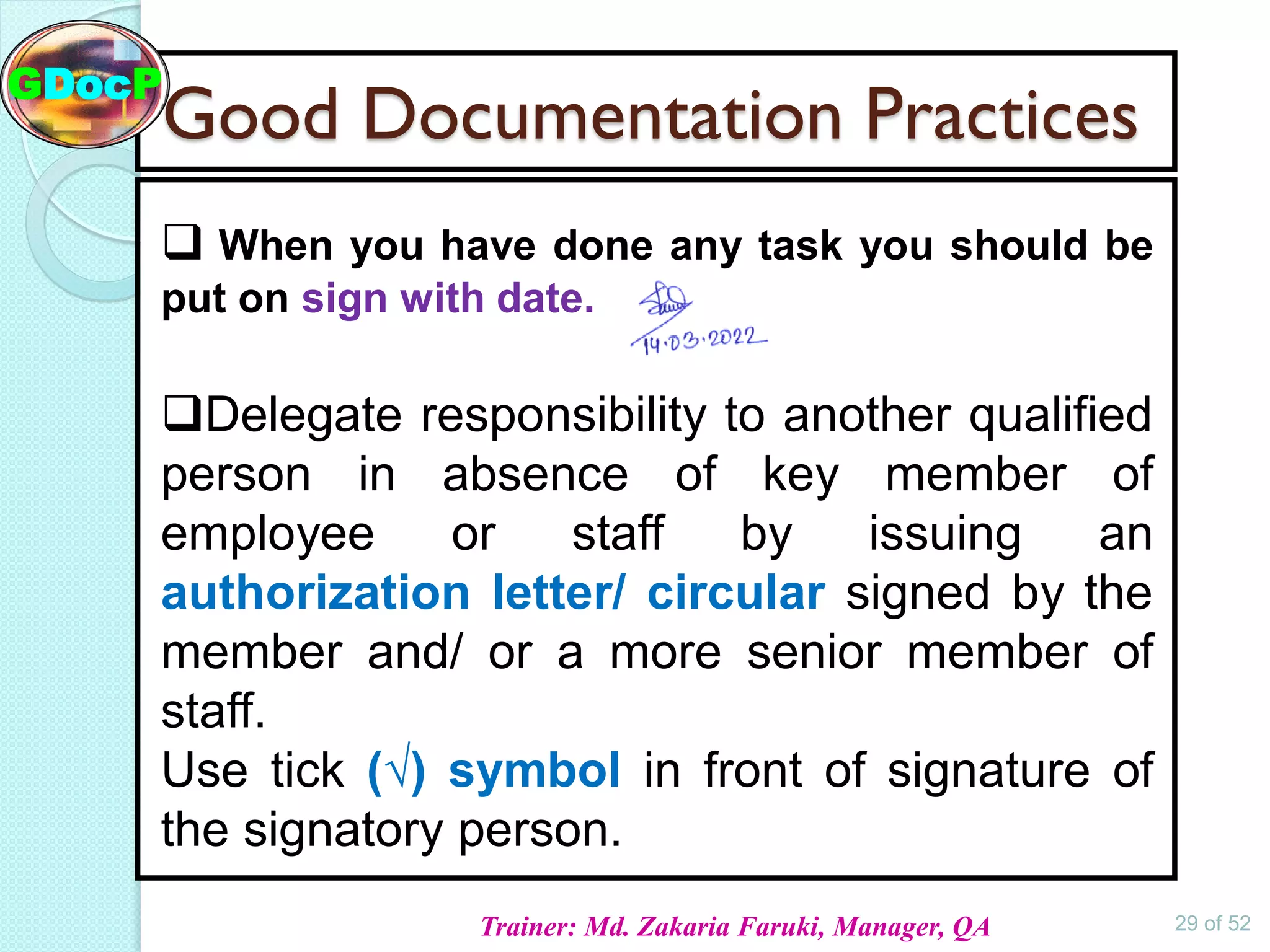
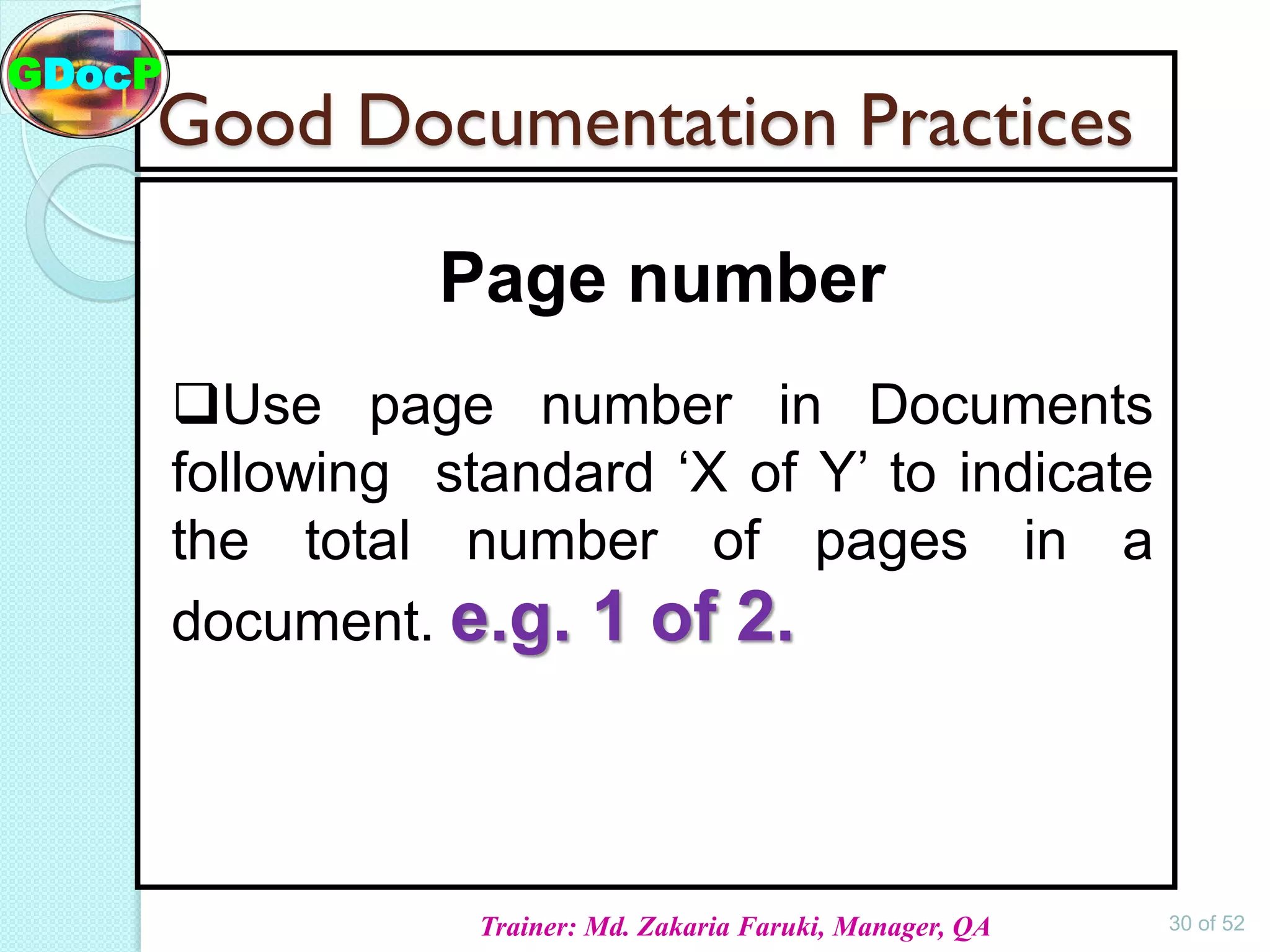
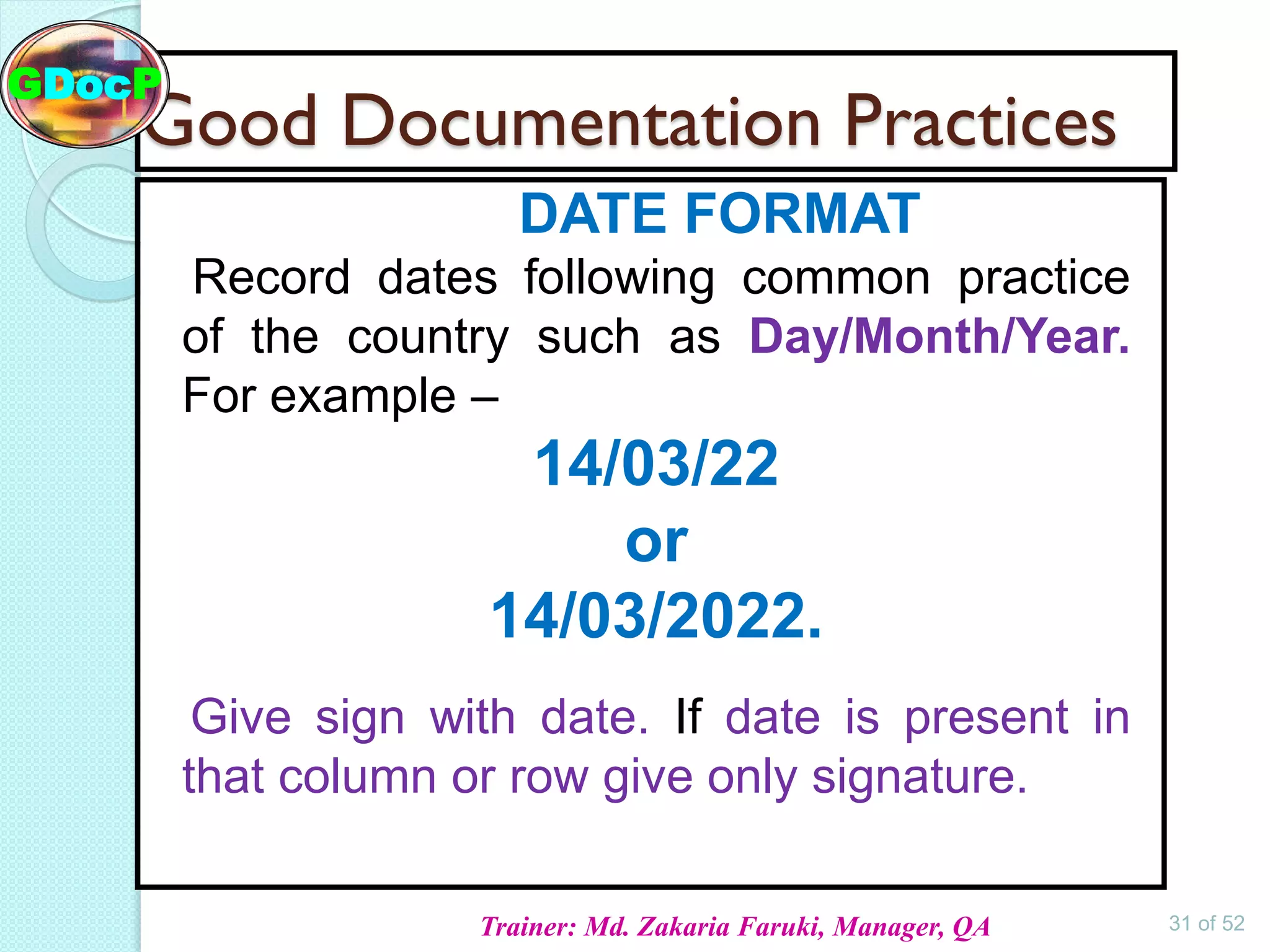
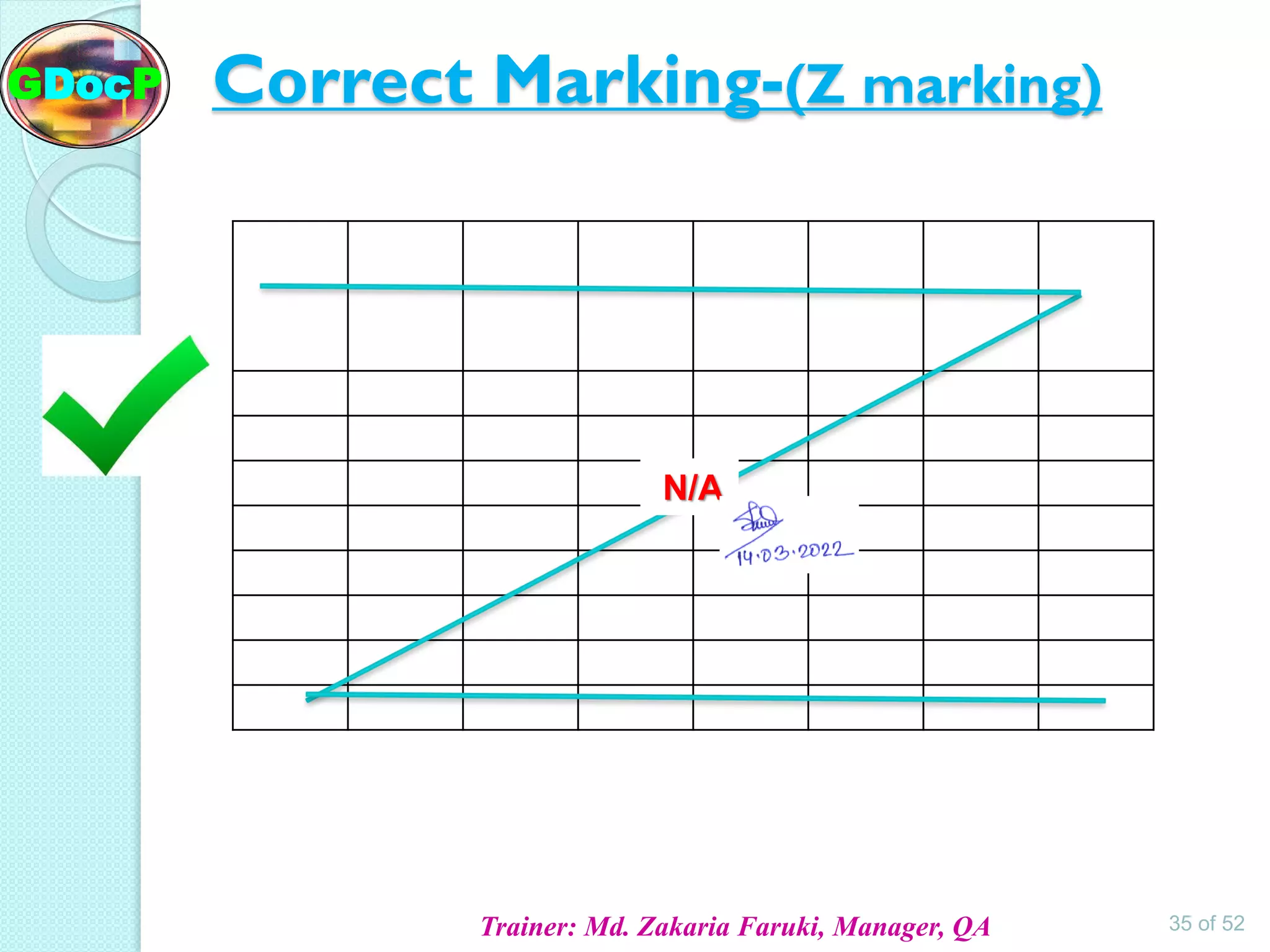
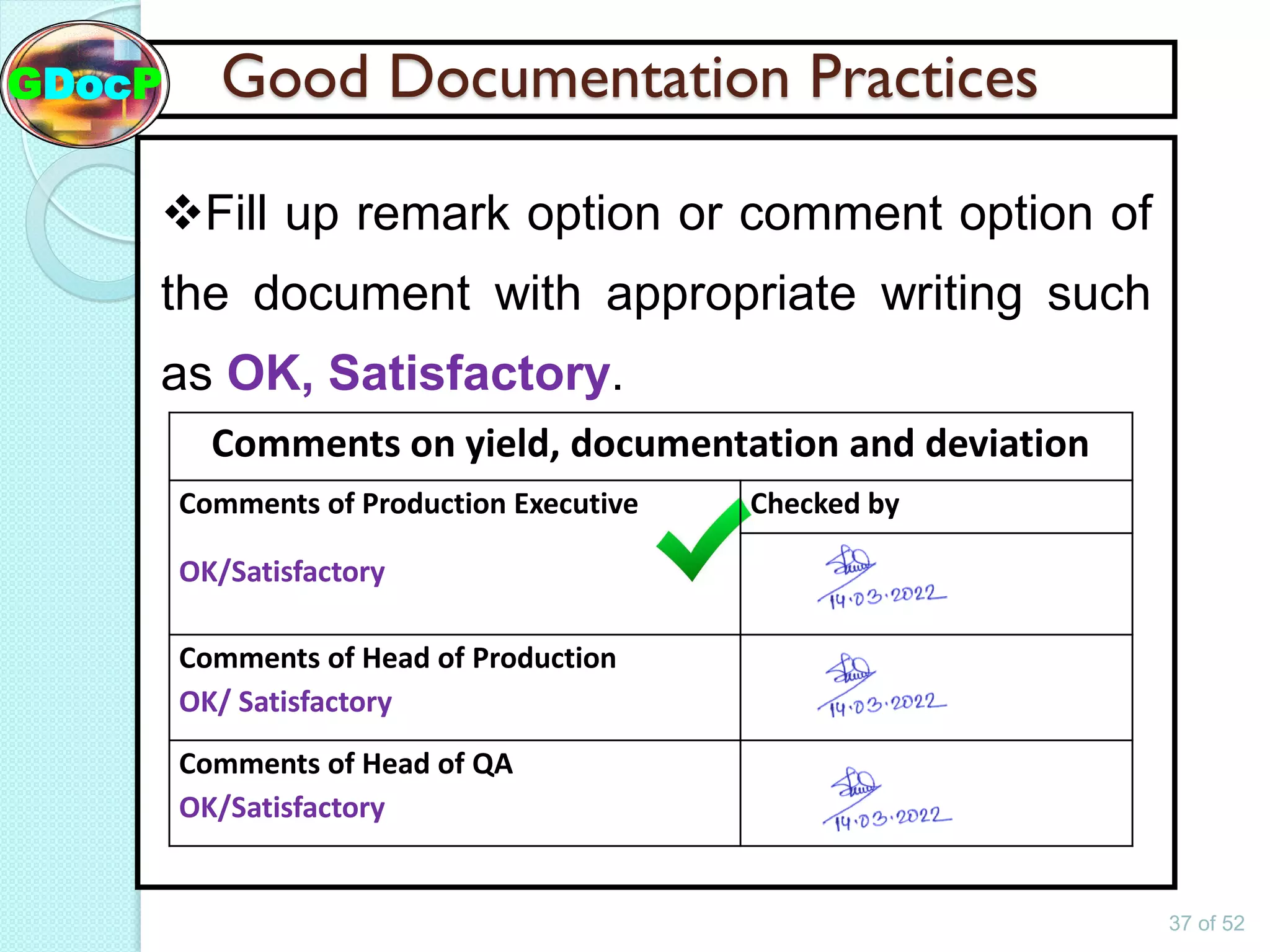
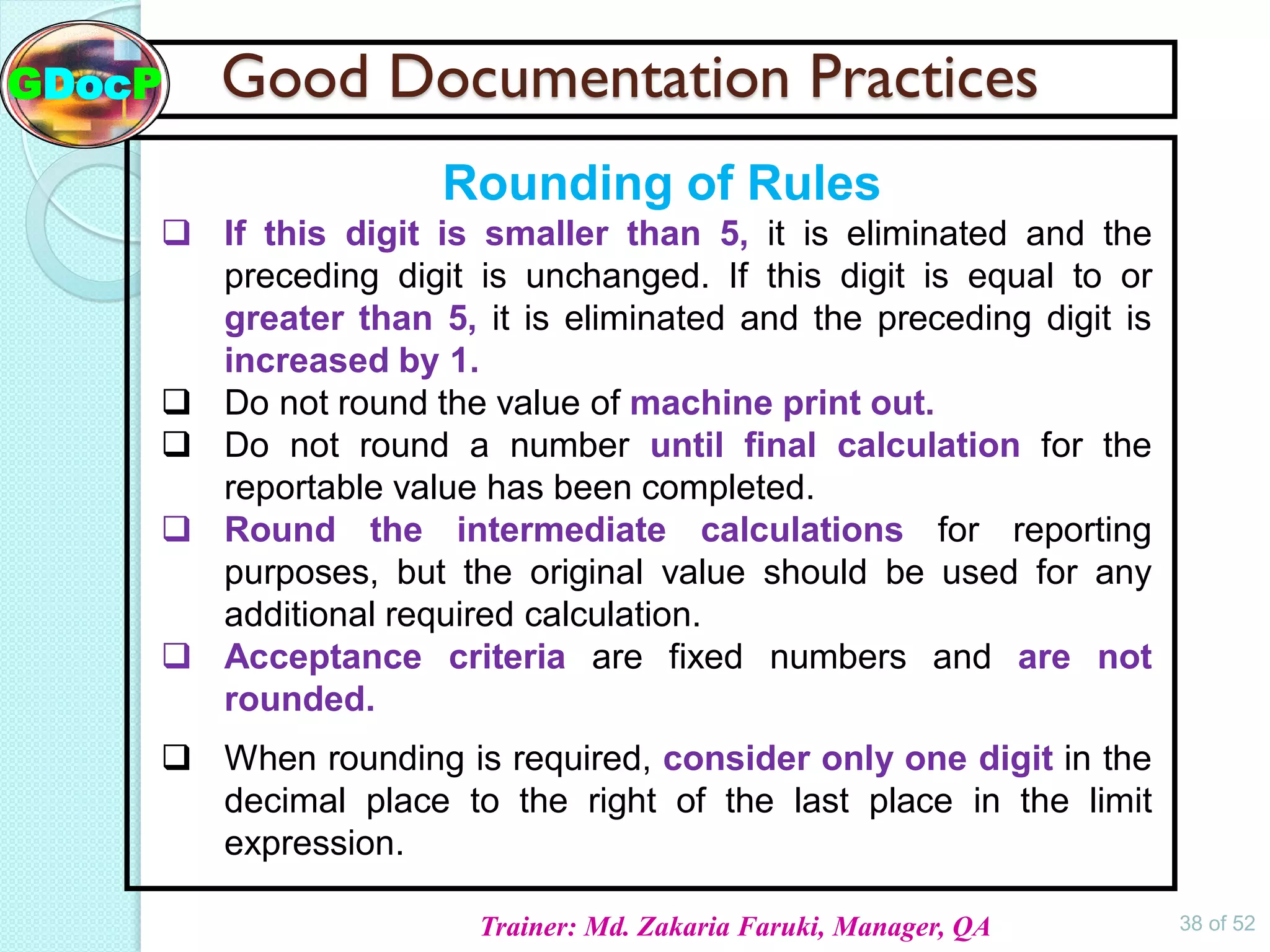
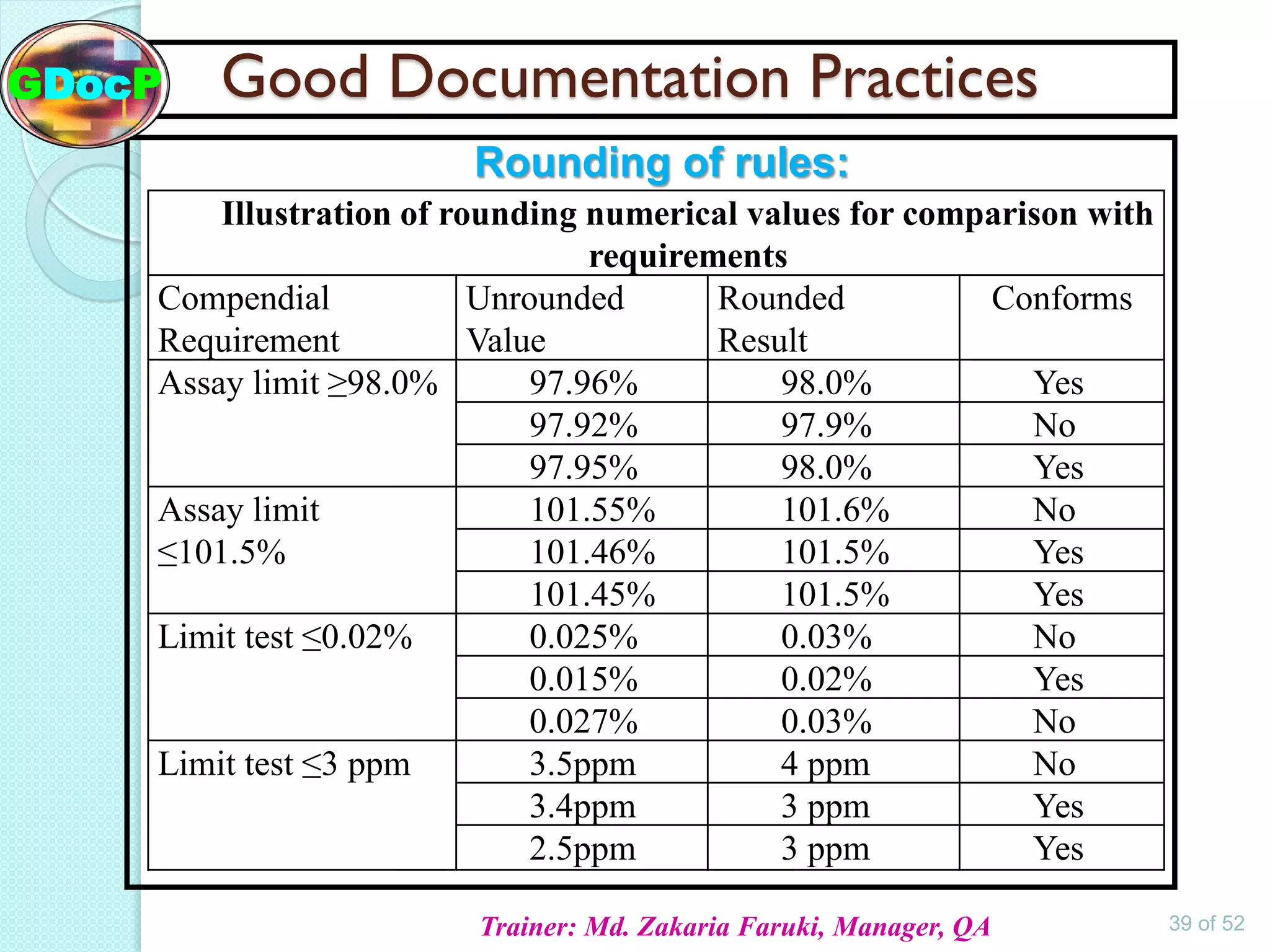
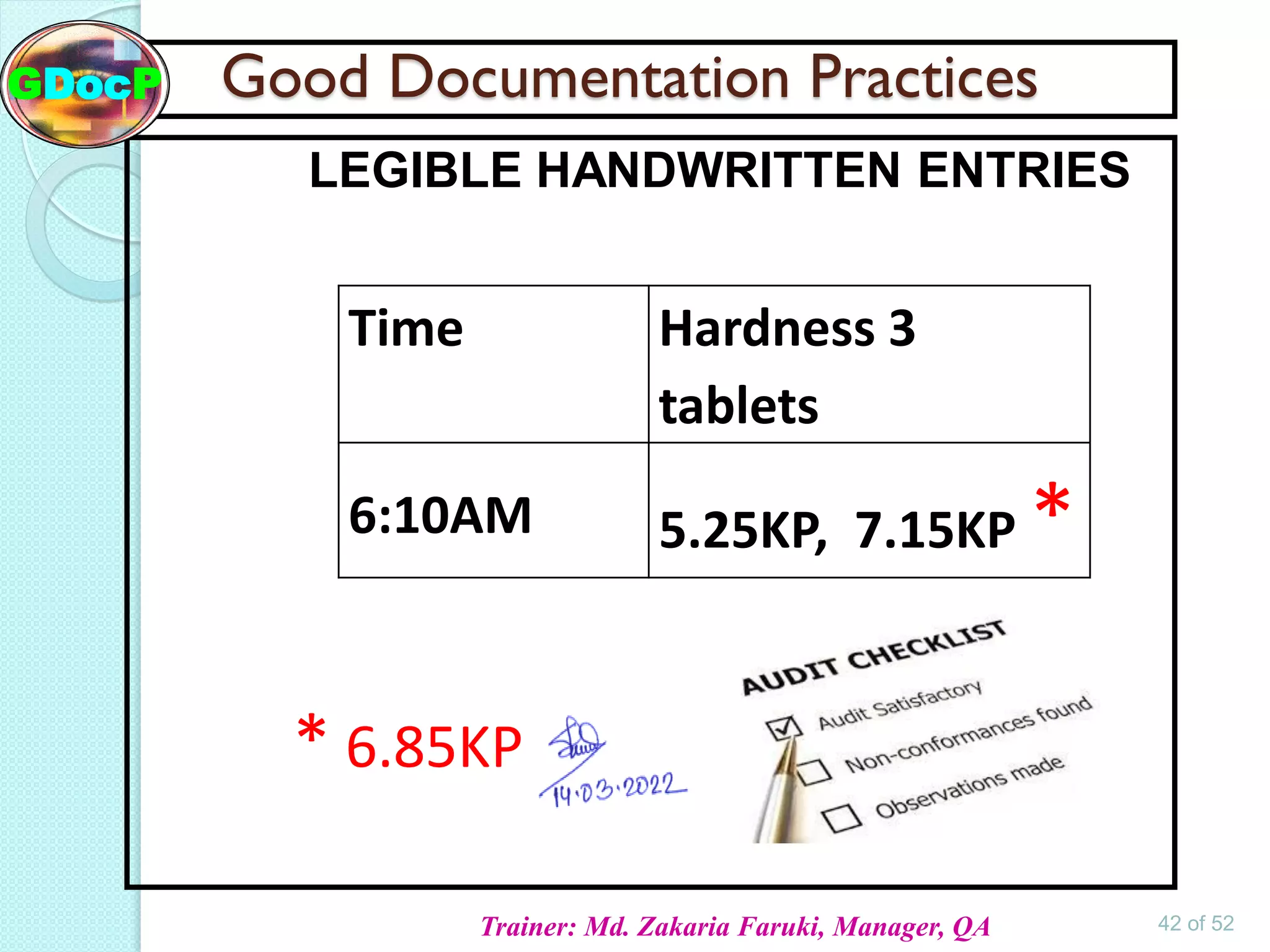
The document outlines Good Documentation Practice (GDP) in the pharmaceutical industry, emphasizing the importance of creating and maintaining accurate, traceable, and legible documents. It details the systematic procedures for documentation, including preparation, review, and approval, as well as the characteristics of good documents and records. Additionally, it provides guidance on proper documentation practices, error correction methods, and regulatory requirements.