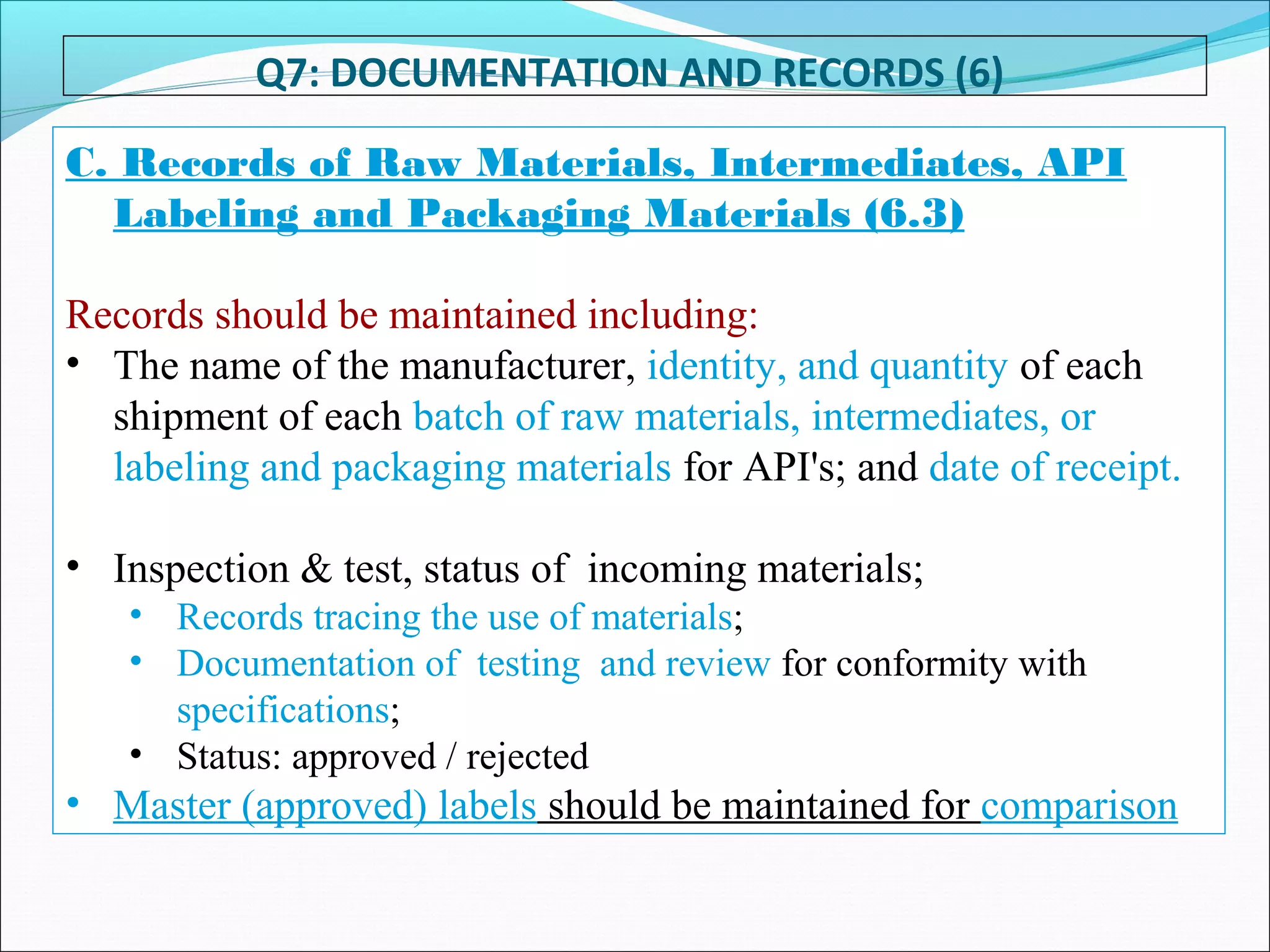
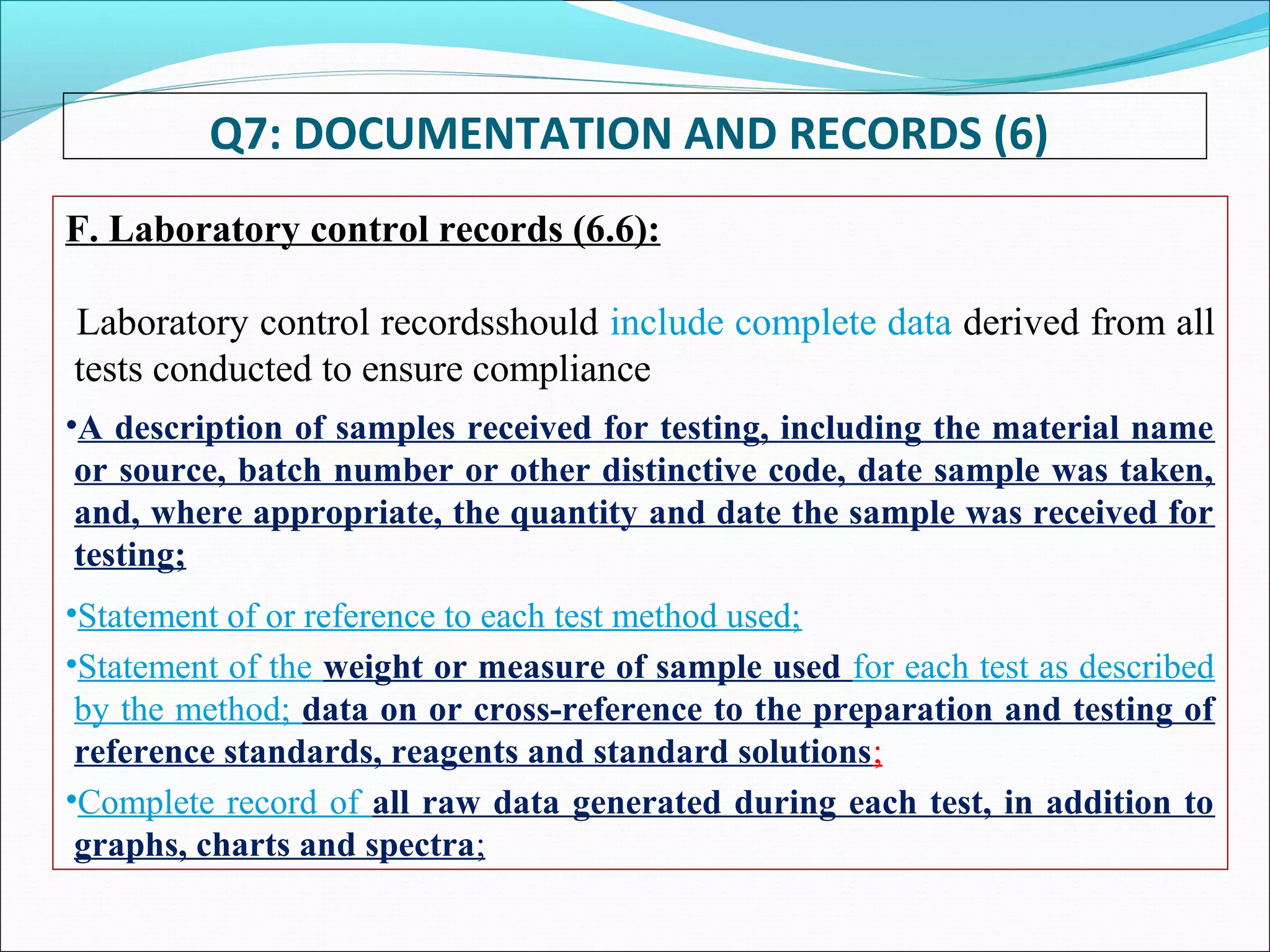
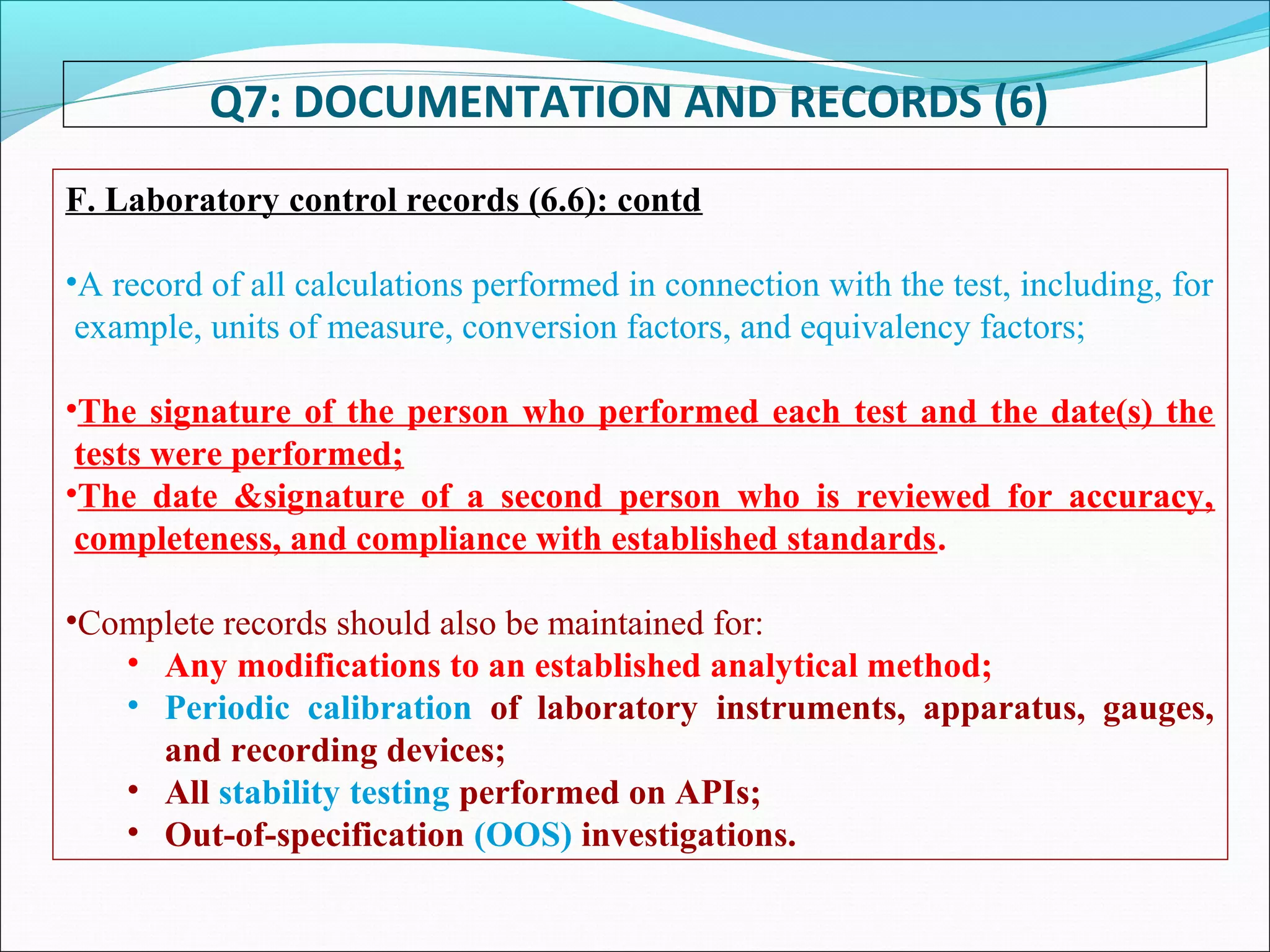
Good Documentation Practice is important to ensure activities are properly documented. GDP defines documents as instructions that guide how activities are executed, while records provide evidence activities were performed. Documentation must be accurate, concise, legible, traceable, contemporaneous, enduring and accessible. It is important to never falsify, obliterate or improperly correct information in records. All activities should be fully documented at the time they are performed. Reviews are necessary to check for completeness and compliance. Maintaining proper documentation is important for regulatory compliance, building confidence, meeting requirements, and solving problems.