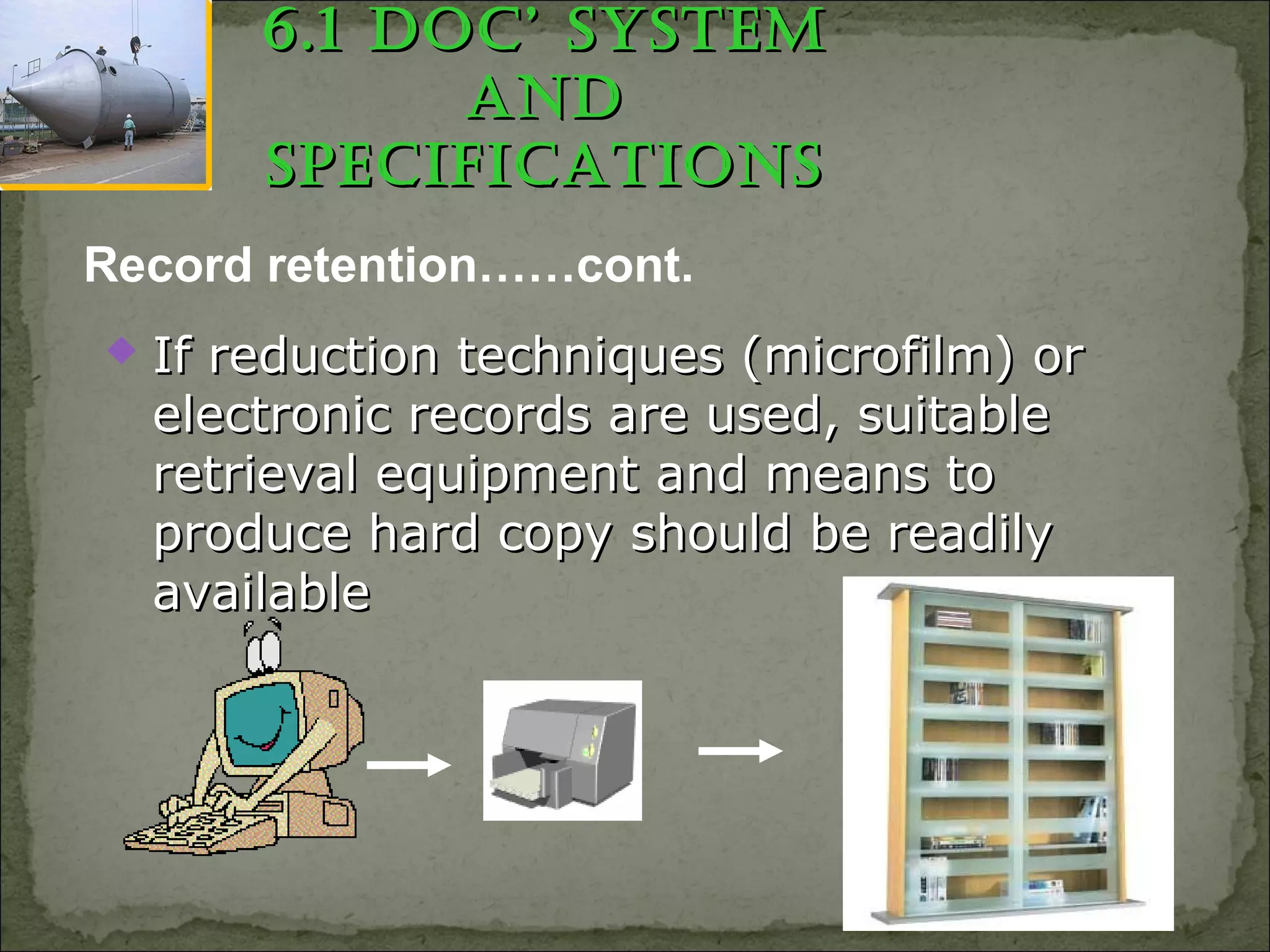
The document outlines Good Manufacturing Practices (GMP) for the documentation of Active Pharmaceutical Ingredients (APIs). It emphasizes the necessity of maintaining accurate records and details various types of documents required, such as batch production records, lab control records, and master production instructions. The guidance stresses the importance of proper documentation practices to ensure product quality and compliance with regulatory standards.