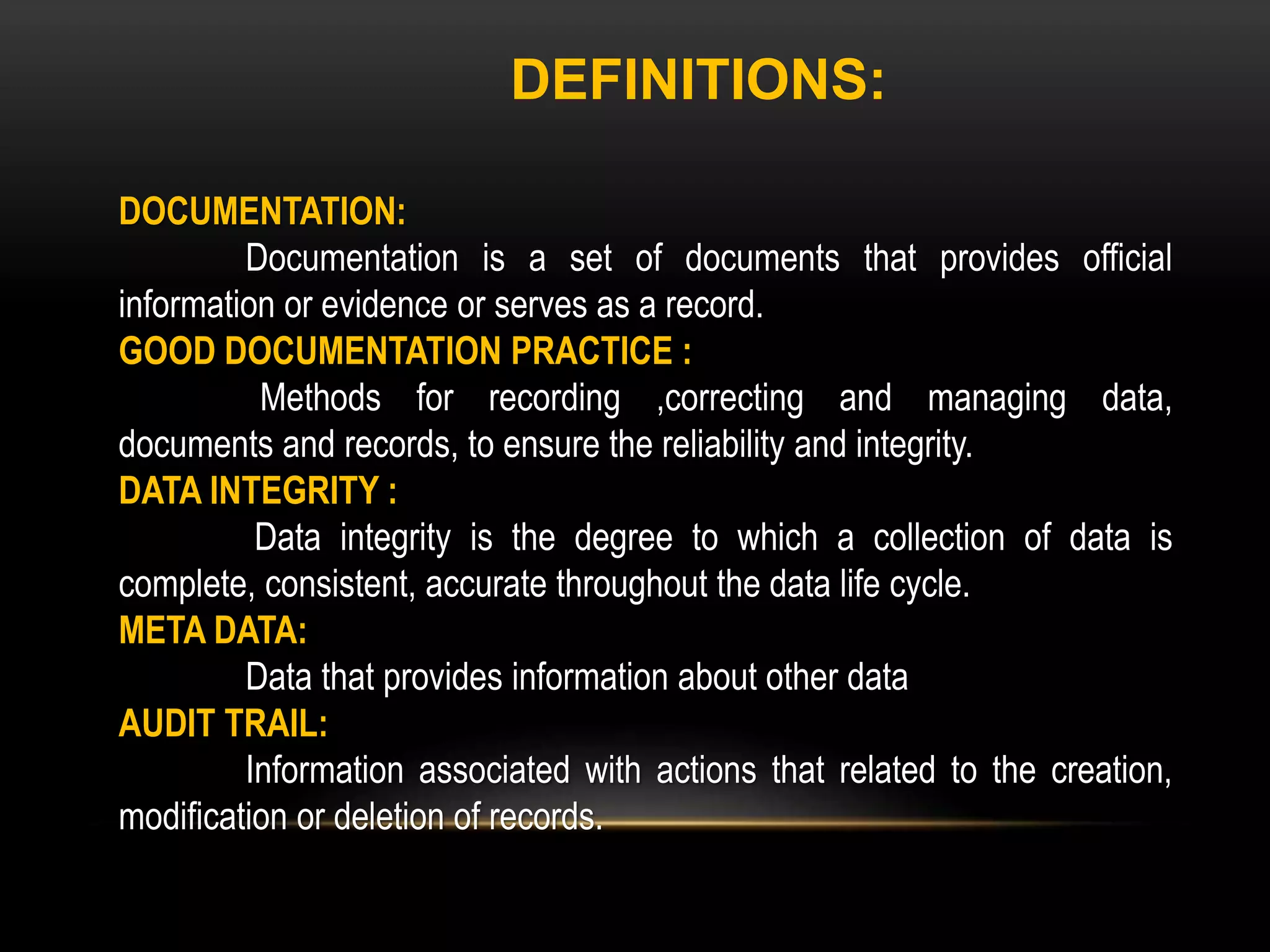
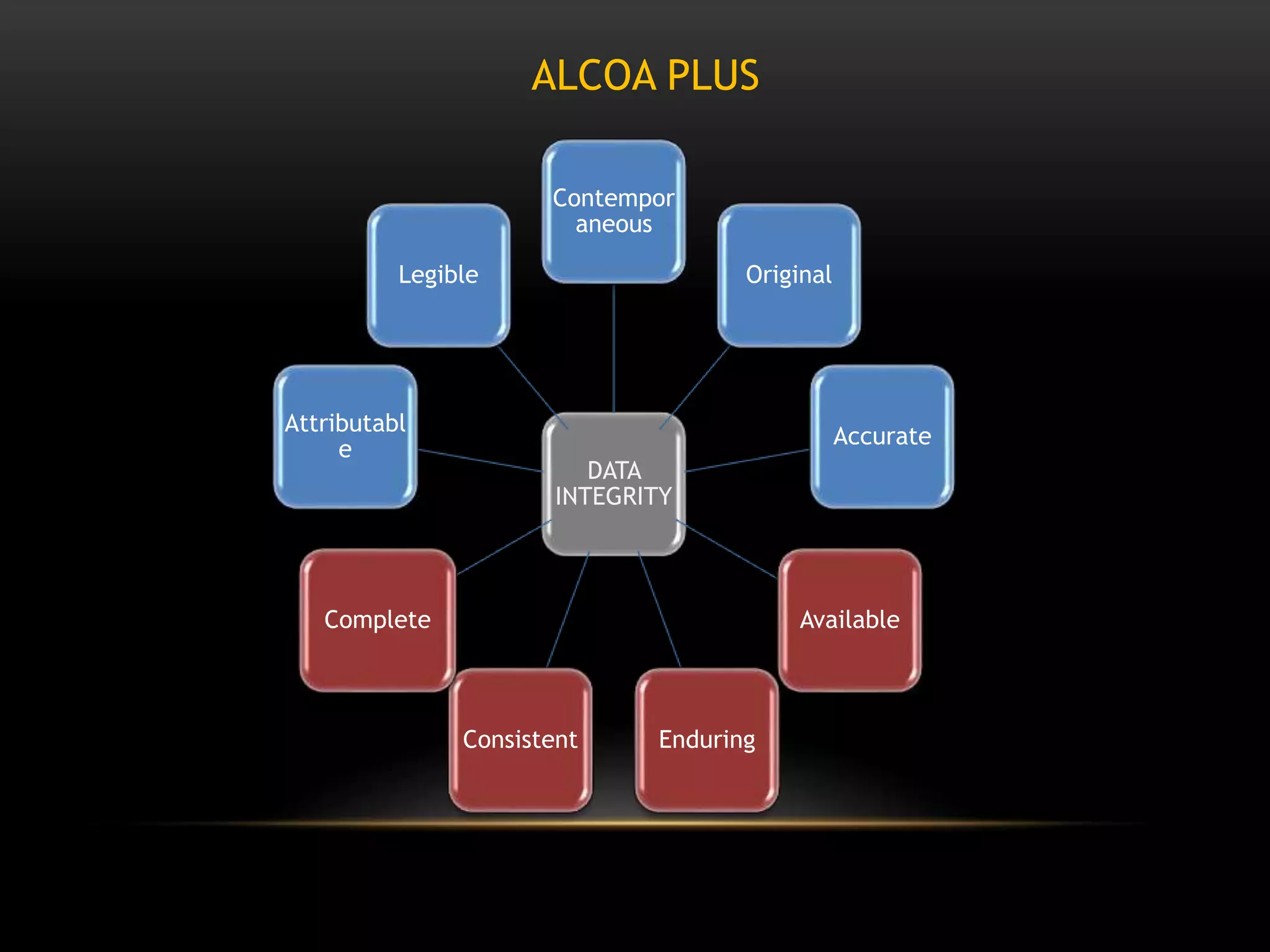
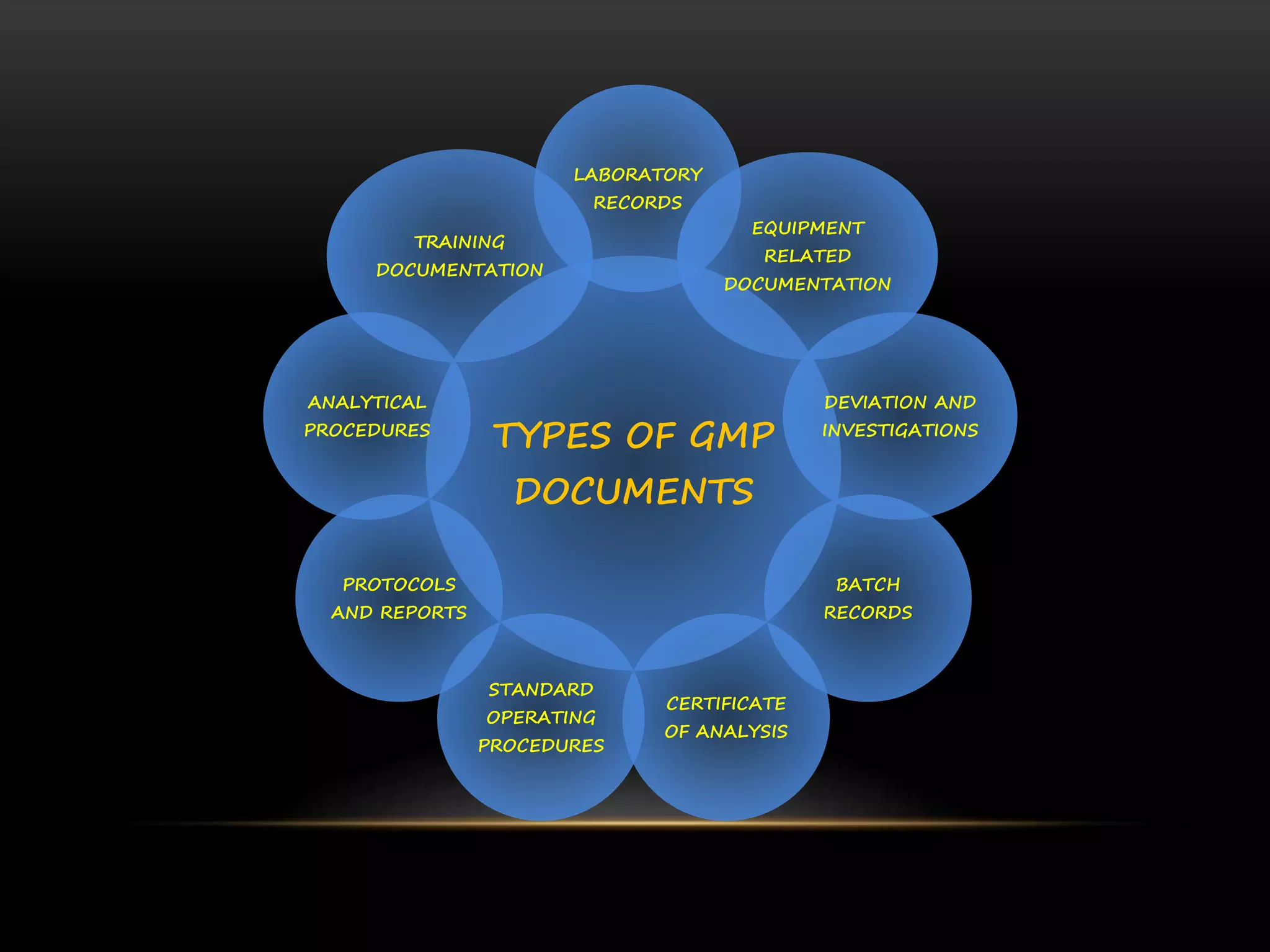
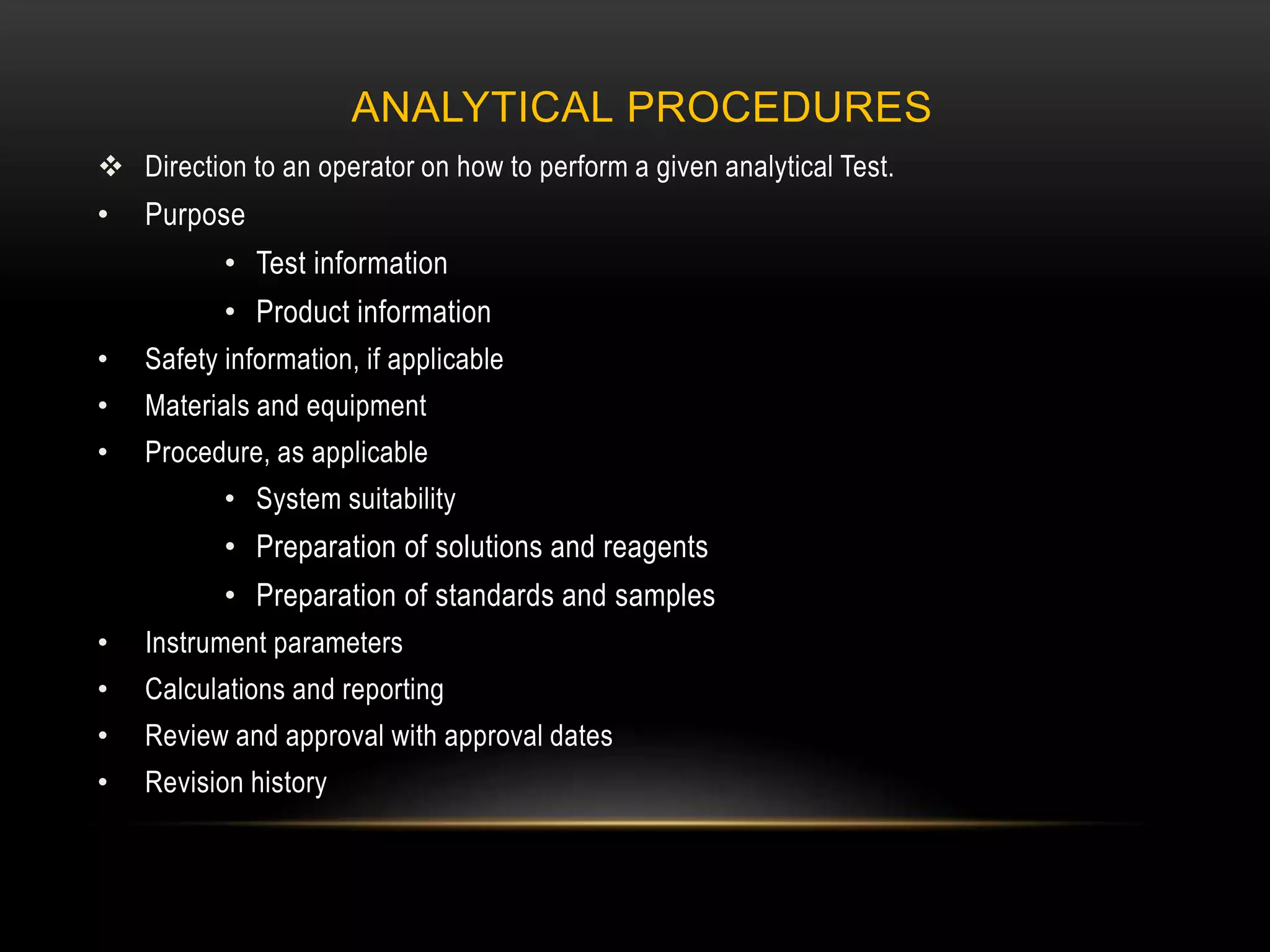
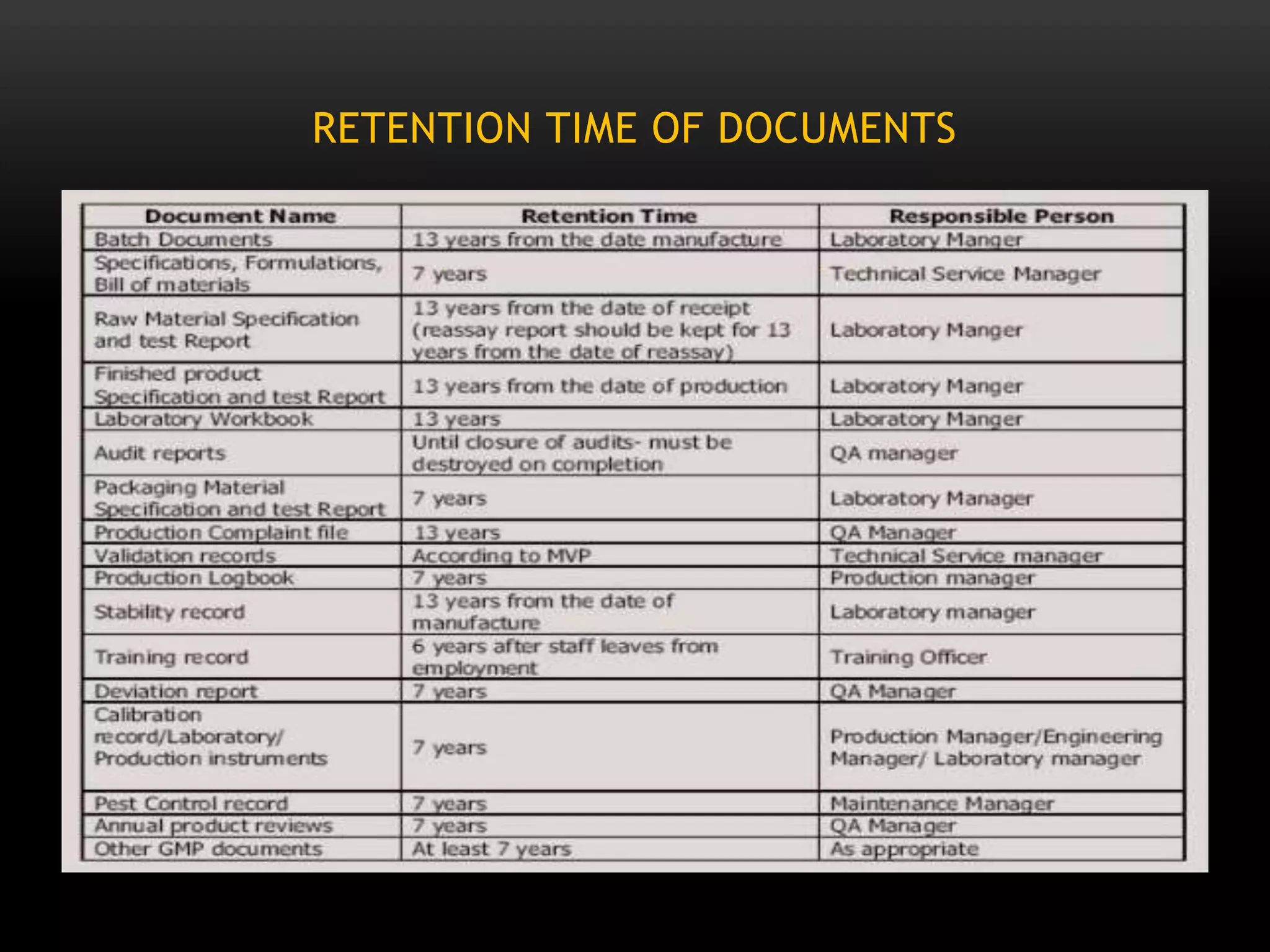
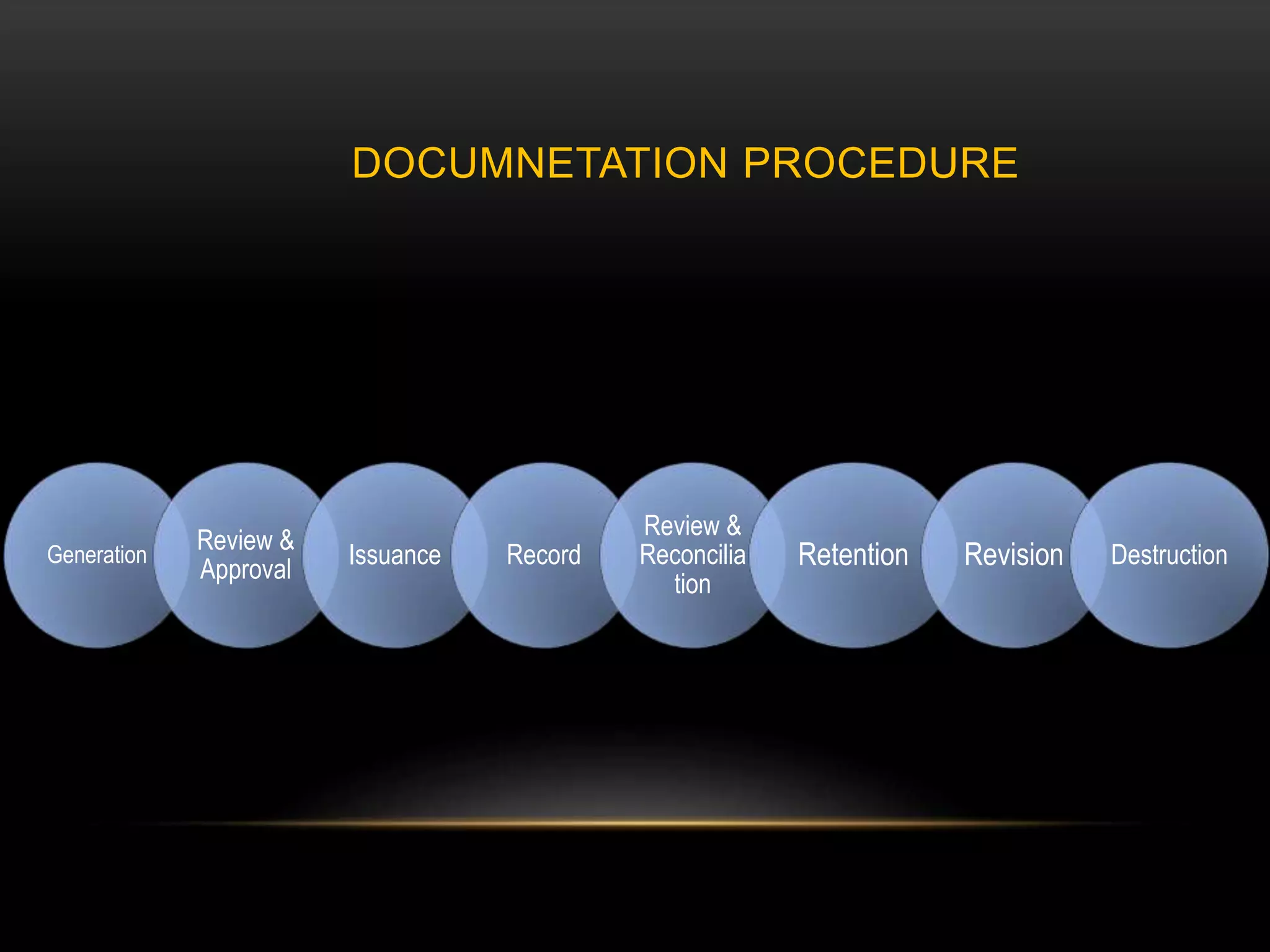
This document defines key terms related to documentation practices for Good Manufacturing Practices (GMP) and outlines the principles and types of documents required by GMP. It discusses documentation requirements for laboratories, equipment, deviations, batch records, certificates of analysis, standard operating procedures, protocols, training, and retention of documents. The key aspects of GMP documentation are that it must be attributable, legible, contemporaneous, original, accurate, complete, consistent, enduring, and available.