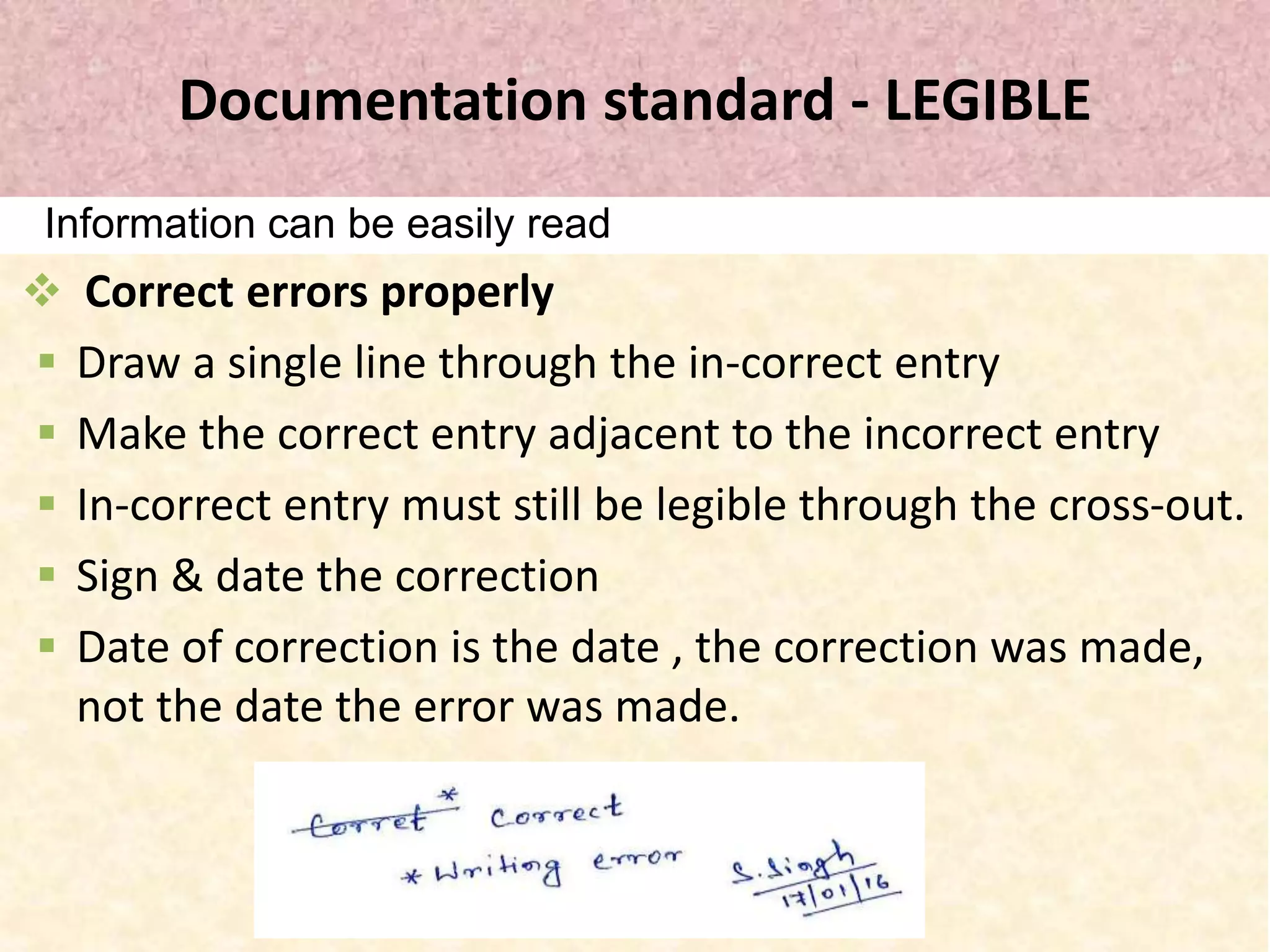
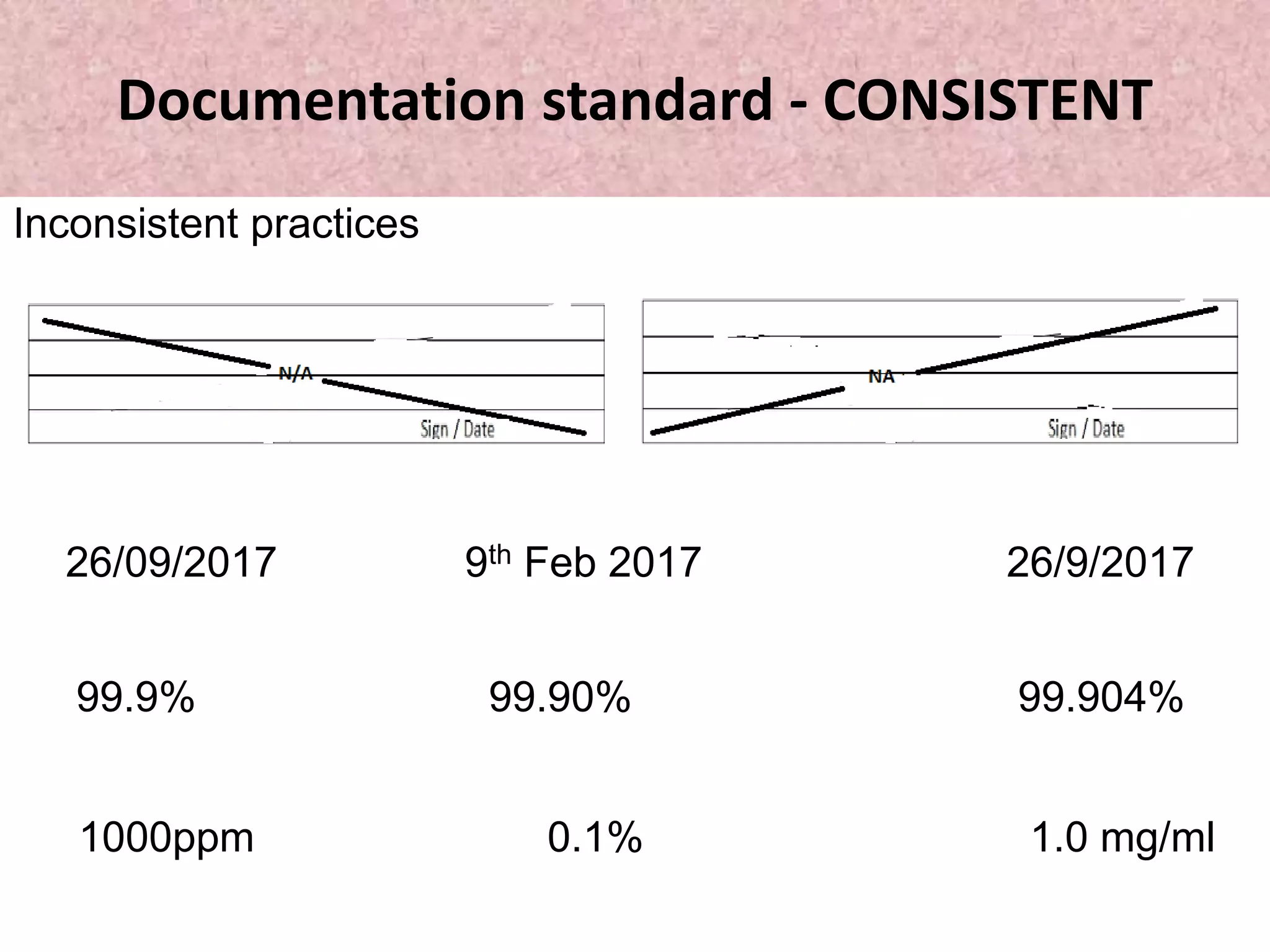
This document discusses good documentation practices (GDP) in the pharmaceutical industry. GDP describes standards for creating and maintaining documentation to ensure regulatory compliance, customer requirements are met, and documentation errors do not cause safety, legal or regulatory issues. Proper GDP is important as documentation serves as an advertisement of how committed and sincere a company is. GDP covers documentation for various organizational functions and requires documentation be permanent, legible, accurate, prompt, clear, consistent, complete, direct, truthful, current and traceable. The document outlines specific standards and steps for GDP including proper recording, corrections, signatures and reviews.