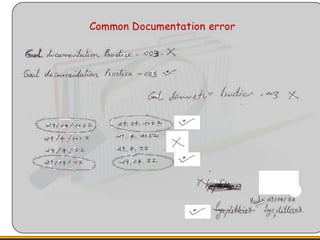
The document outlines the importance of Good Manufacturing Practice (GMP) documentation in ensuring compliance with regulatory requirements, detailing key features and purposes of effective documentation practices. It emphasizes systematic recording, review, and approval processes for documentation, highlighting the necessity of maintaining accurate records to uphold product quality and legality. The text also addresses common pitfalls in documentation and offers tips for maintaining high standards in recordkeeping.