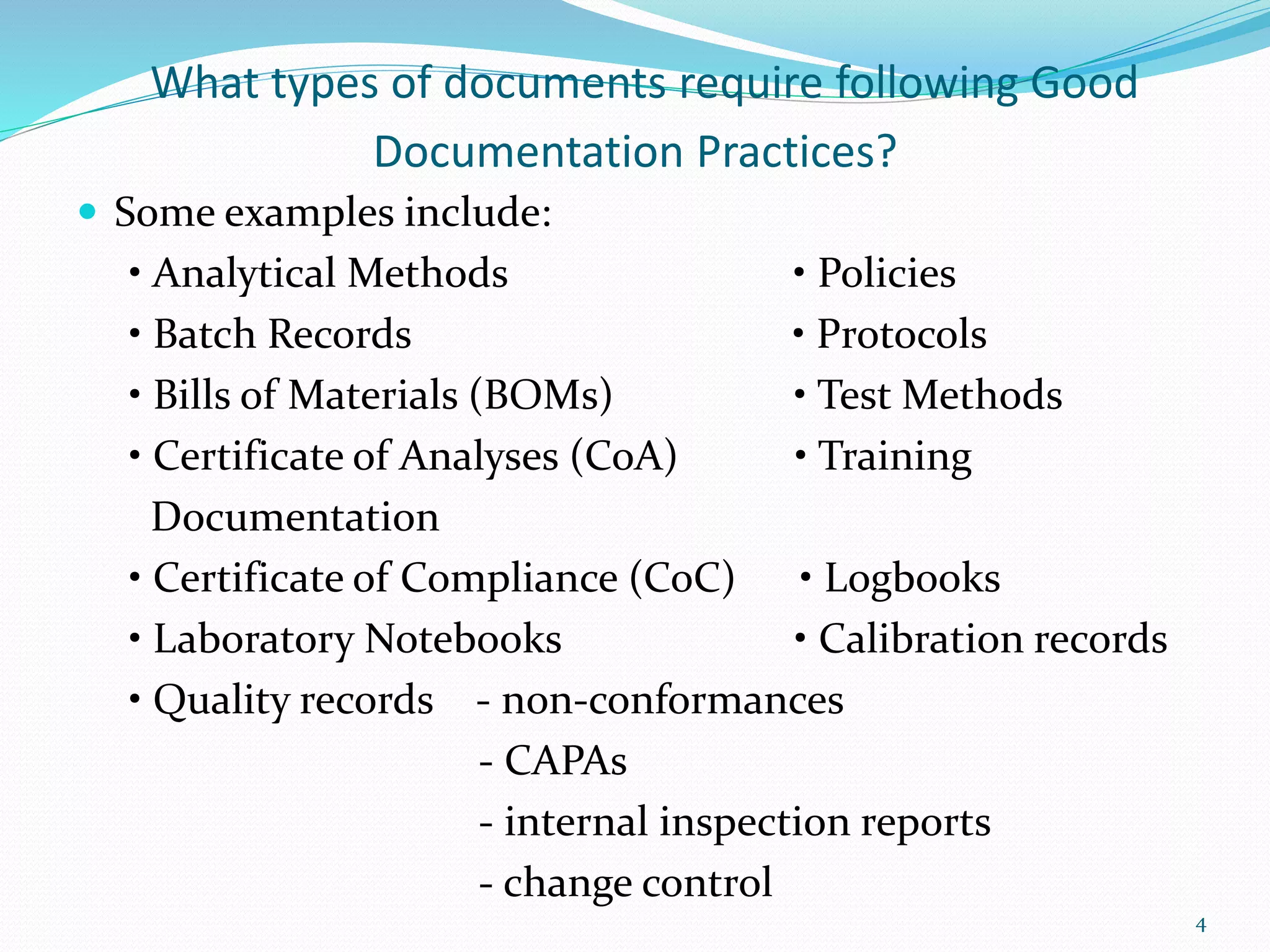
This document discusses Good Documentation Practices (GDP) which are an essential part of quality assurance according to WHO. GDPs apply to anyone documenting cGMP activities. Key aspects of GDP include ensuring documentation is true, accurate, timely and legible. Documents that require following GDP include batch records, standard operating procedures, validation documents, and quality records. Signatures on GMP documents confirm identity and responsibility. Documentation serves to define specifications and procedures, ensure regulatory compliance, and provide an audit trail. Basic GDP requirements include contemporaneous recording, signatures and dates, use of English, no corrections, and no backdating.