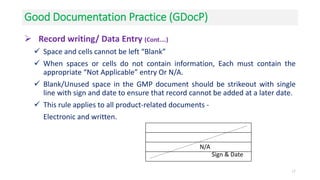
The document provides guidance on good documentation practices for pharmaceutical manufacturing. It discusses the importance of documentation and outlines key requirements for documents and records to ensure data integrity. These include maintaining batch records, equipment cleaning records, raw material records, production instructions, laboratory testing records, and conducting batch record reviews. Documentation must be accurate, contemporaneous, attributable, complete, consistent, enduring and readily available. All activities should be properly documented at the time they are performed and any changes must be justified and traceable. Good documentation practices are necessary to demonstrate compliance and allow for traceability in the pharmaceutical quality system.