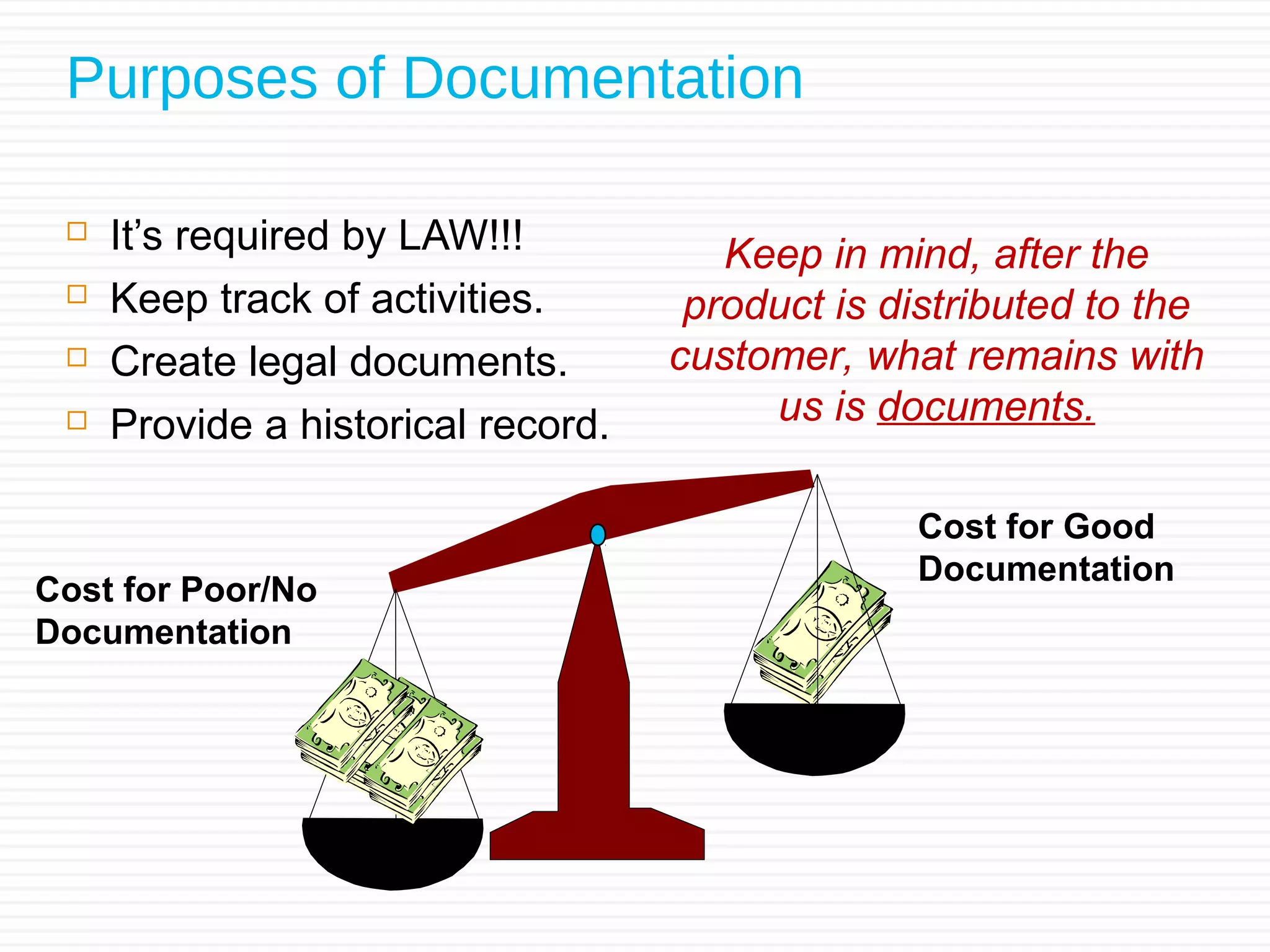
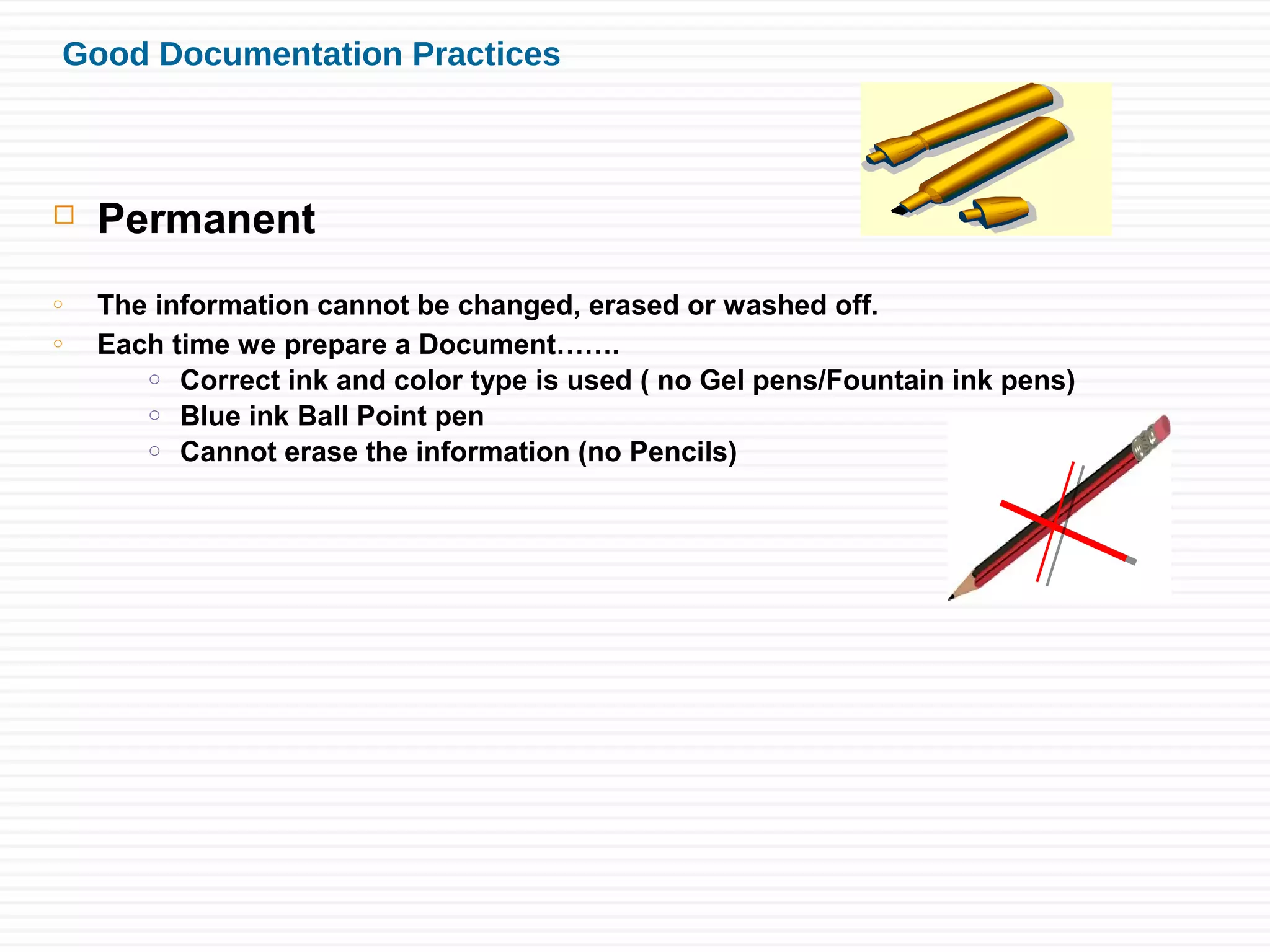
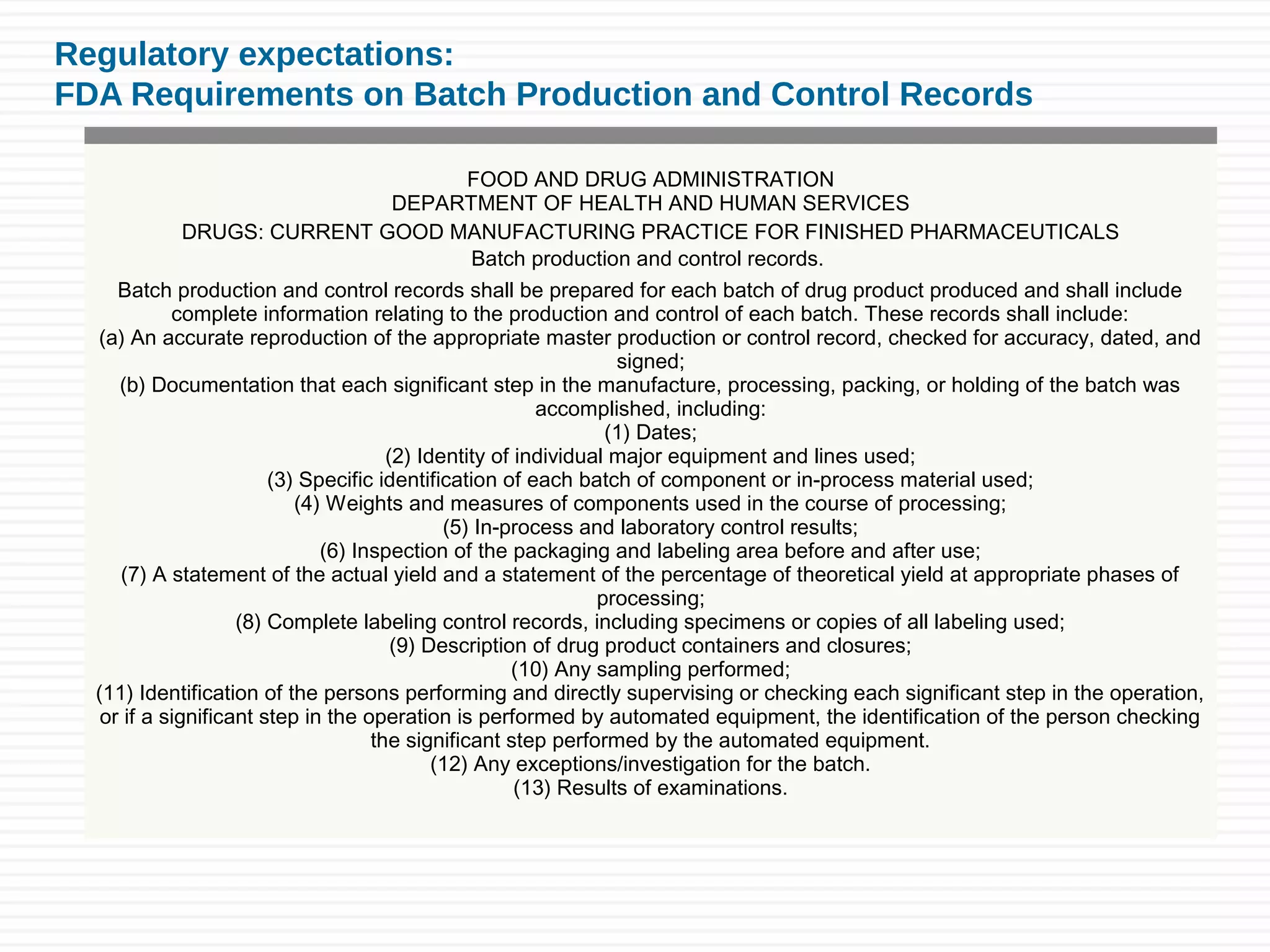
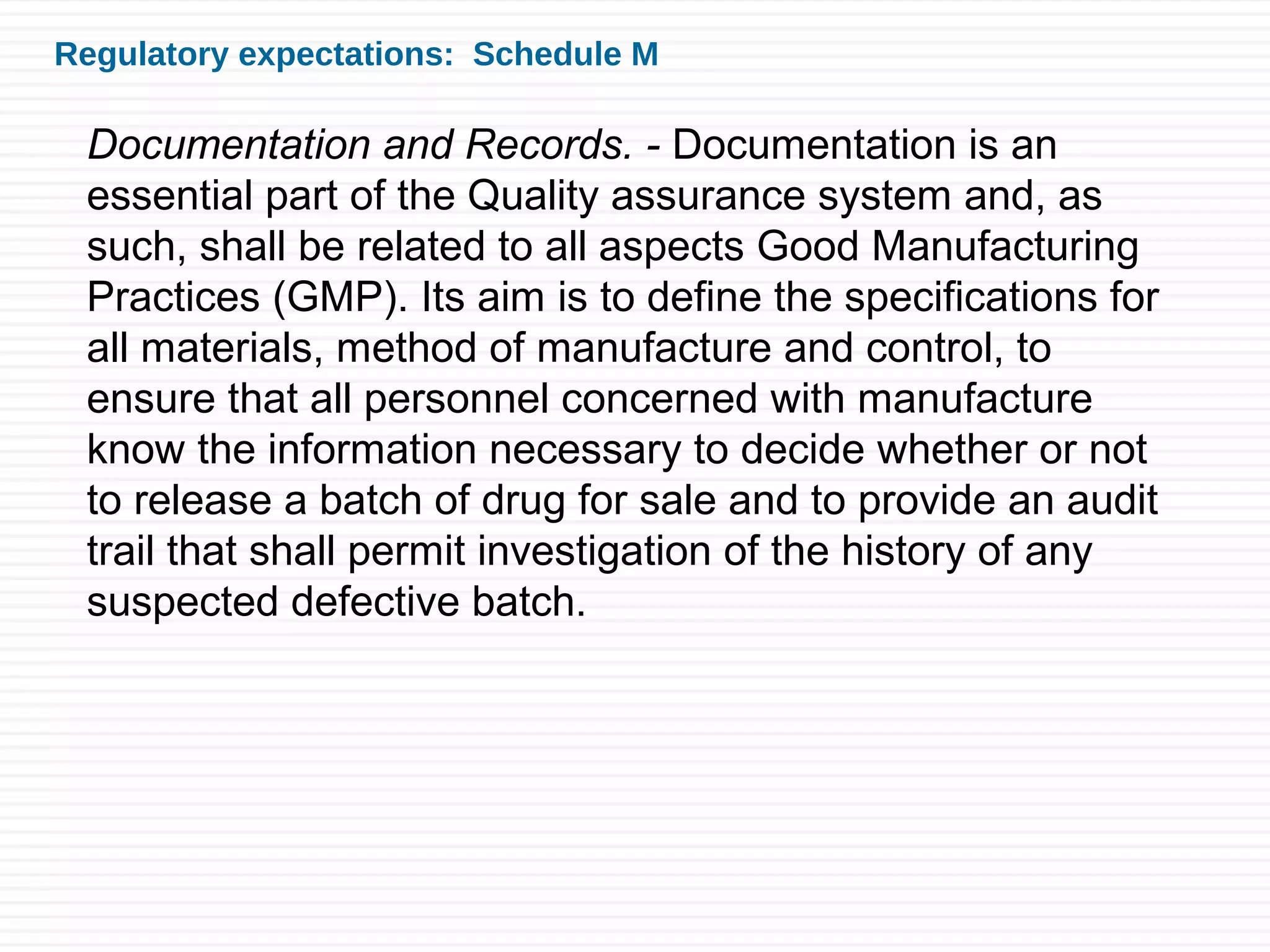
Sushant Sardana's objective is to raise awareness of the importance of documentation for pharmaceutical and healthcare products. Good documentation practices (GDP) are required by law and regulations to demonstrate compliance and provide an audit trail. GDP help ensure documentation is permanent, legible, accurate, prompt, clear, consistent, complete, direct, and truthful. Major errors in documentation must be properly corrected and explained. If an activity is not documented, it did not happen according to regulatory standards.