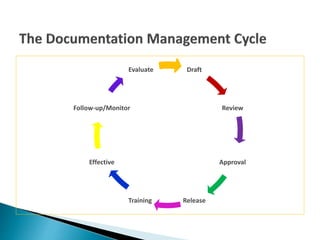
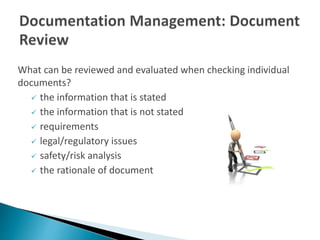
The document outlines the importance of documentation in regulated industries, detailing the requirements for effective documentation practices according to ISO 9001:2008. It emphasizes the need for clear procedures, roles, and review processes to ensure quality and compliance, and mentions the various documents that should be maintained. Additionally, it encourages utilizing resources such as webinars for further training on good documentation practices.