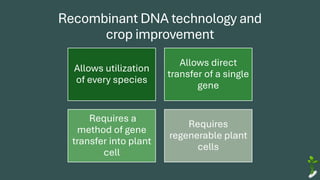
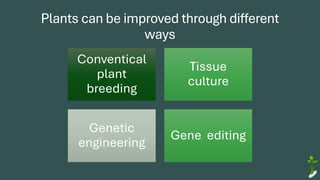
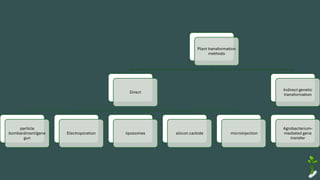
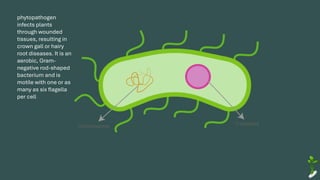
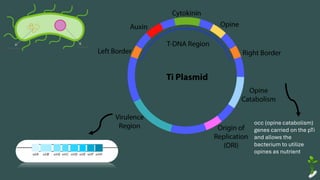
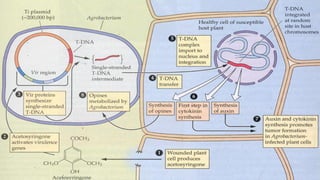
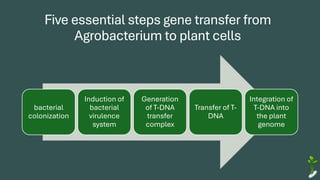
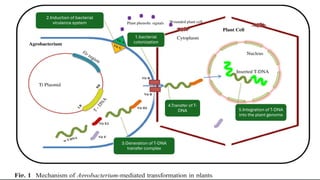
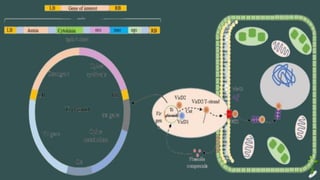
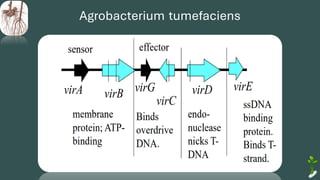
The document discusses gene transfer in plants through recombinant DNA technology, highlighting various methods, including agrobacterium-mediated transformation. It provides details on the mechanisms by which agrobacteria colonize plant cells and transfer T-DNA, resulting in genetic modifications. Additionally, it outlines the advantages of different bacterial species and plasmids involved in this process for crop improvement.









![References
• [1]M. Naseem et al., “The effects of ginkgo biloba leaf extract on metabolic disturbances associated to alloxan-
induced diabetic rats,” J. Anim. Plant Sci., vol. 26, no. 3, pp. 627–635, 2016, doi: 10.1128/MMBR.67.1.16.
• [2]P. B. Moses, Gene Transfer Methods Applicable to Agricultural Organisms. 1987.
• [3]S. Mehrotra and V. Goyal, “Agrobacterium-Mediated Gene Transfer in Plants and Biosafety Considerations,” Appl.
Biochem. Biotechnol., vol. 168, no. 7, pp. 1953–1975, 2012, doi: 10.1007/s12010-012-9910-6.
• [4]G. Keshavareddy, A. R. V. Kumar, and V. S. Ramu, “Methods of Plant Transformation- A Review,” Int. J. Curr.
Microbiol. Appl. Sci., vol. 7, no. 07, pp. 2656–2668, 2018, doi: 10.20546/ijcmas.2018.707.312.
• [5]S. Rahangdale, J. Nehru, K. Vishwavidyalaya, Y. Singh, J. Nehru, and K. Vishwavidyalaya, “Advances in Biological
Sciences and Biotechnology,” Adv. Biol. Sci. Biotechnol., no. January, 2020, doi: 10.22271/int.book.11.
• [6]W. Su, M. Xu, Y. Radani, and L. Yang, “Technological Development and Application of Plant Genetic
Transformation,” Int. J. Mol. Sci., vol. 24, no. 13, 2023, doi: 10.3390/ijms241310646.
• [7]P. J. Larkin, Genetic engineering of plants — agricultural research opportunities and policy concerns, vol. 16, no. 2.
1986. doi: 10.1016/0167-8809(86)90104-0.
• [8]J. R. Zupan and P. Zambryski, “Transfer of T-DNA from,” no. 1 995.
• [9]R. Imai et al., “In planta particle bombardment (IPB): A new method for plant transformation and genome editing,”
Plant Biotechnol., vol. 37, no. 2, pp. 171–176, 2020, doi: 10.5511/PLANTBIOTECHNOLOGY.20.0206A.
• [10]Y. Zhang, Q. Zhang, and Q. J. Chen, “Agrobacterium-mediated delivery of CRISPR/Cas reagents for genome editing
in plants enters an era of ternary vector systems,” Sci. China Life Sci., vol. 63, no. 10, pp. 1491–1498, 2020, doi:
10.1007/s11427-020-1685-9.](https://image.slidesharecdn.com/genetransferinplantsagrobacterium-240326043426-a73fff9d/85/Gene-transfer-in-plants-agrobacterium-pdf-21-320.jpg)