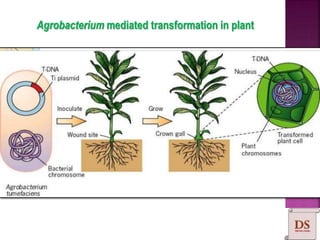
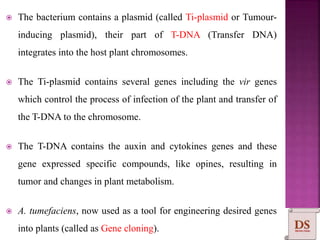
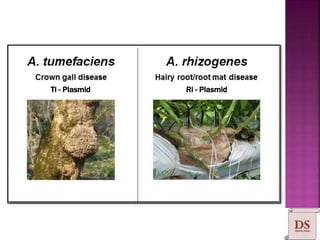
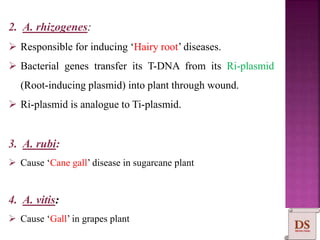
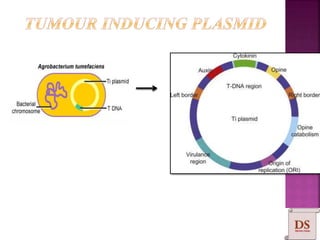
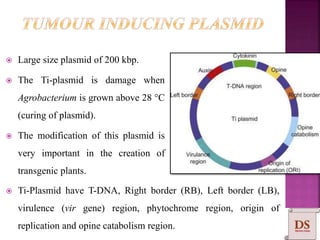
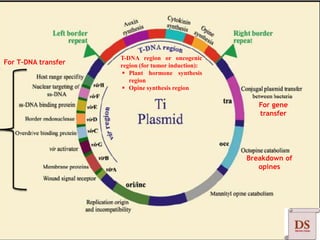
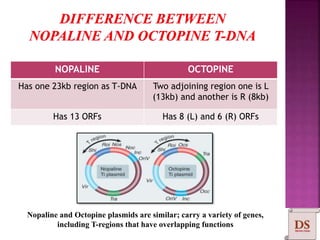
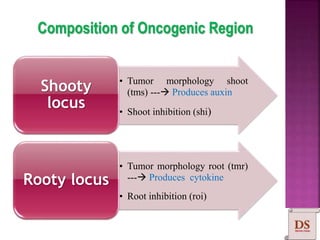
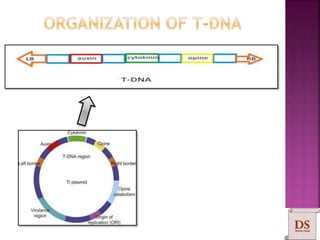
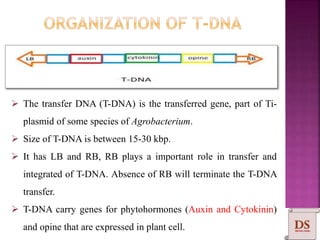
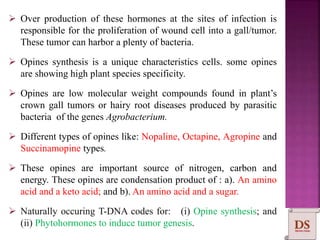
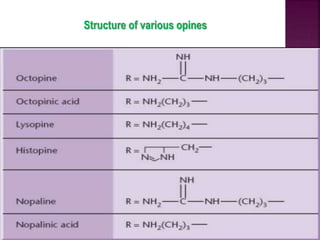
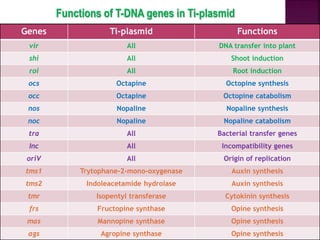
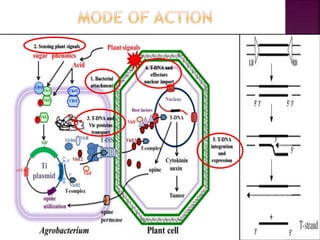
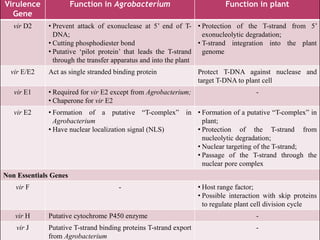
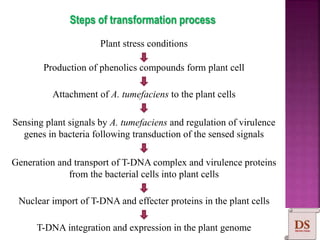
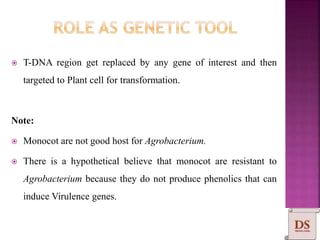
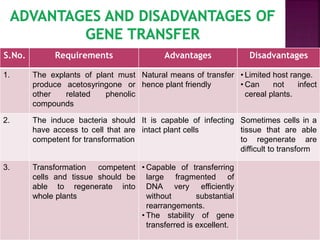
Agrobacterium species, particularly A. tumefaciens and A. rhizogenes, are known for their ability to naturally infect plants, causing diseases and enabling genetic transformation. They utilize ti- and ri-plasmids to transfer T-DNA into the plant genome, which leads to tumor formation and altered plant metabolism through the expression of specific genes. This mechanism has been harnessed for gene cloning and the creation of transgenic plants, though monocots are generally resistant to Agrobacterium due to the lack of inducing phenolic compounds.