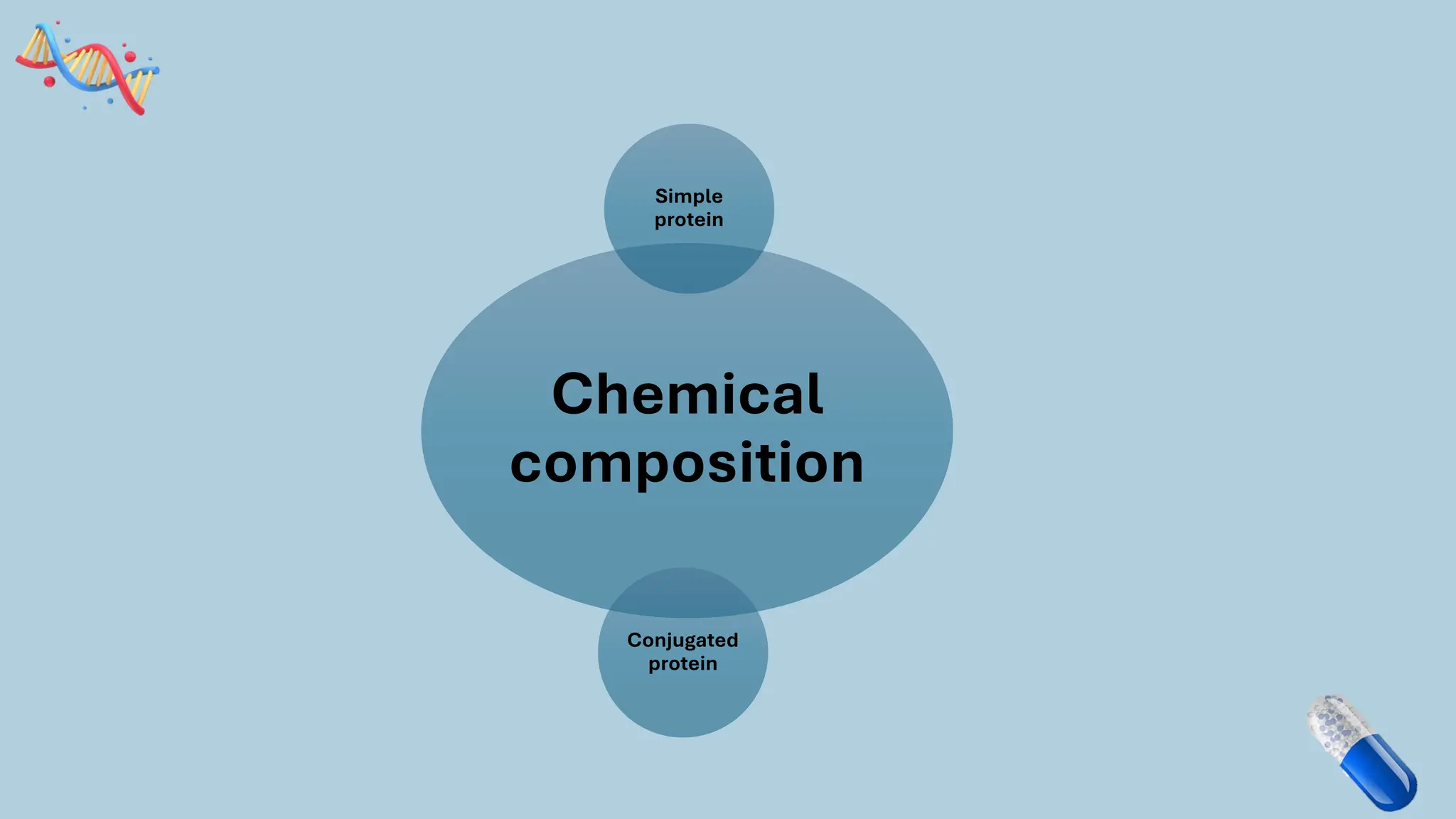
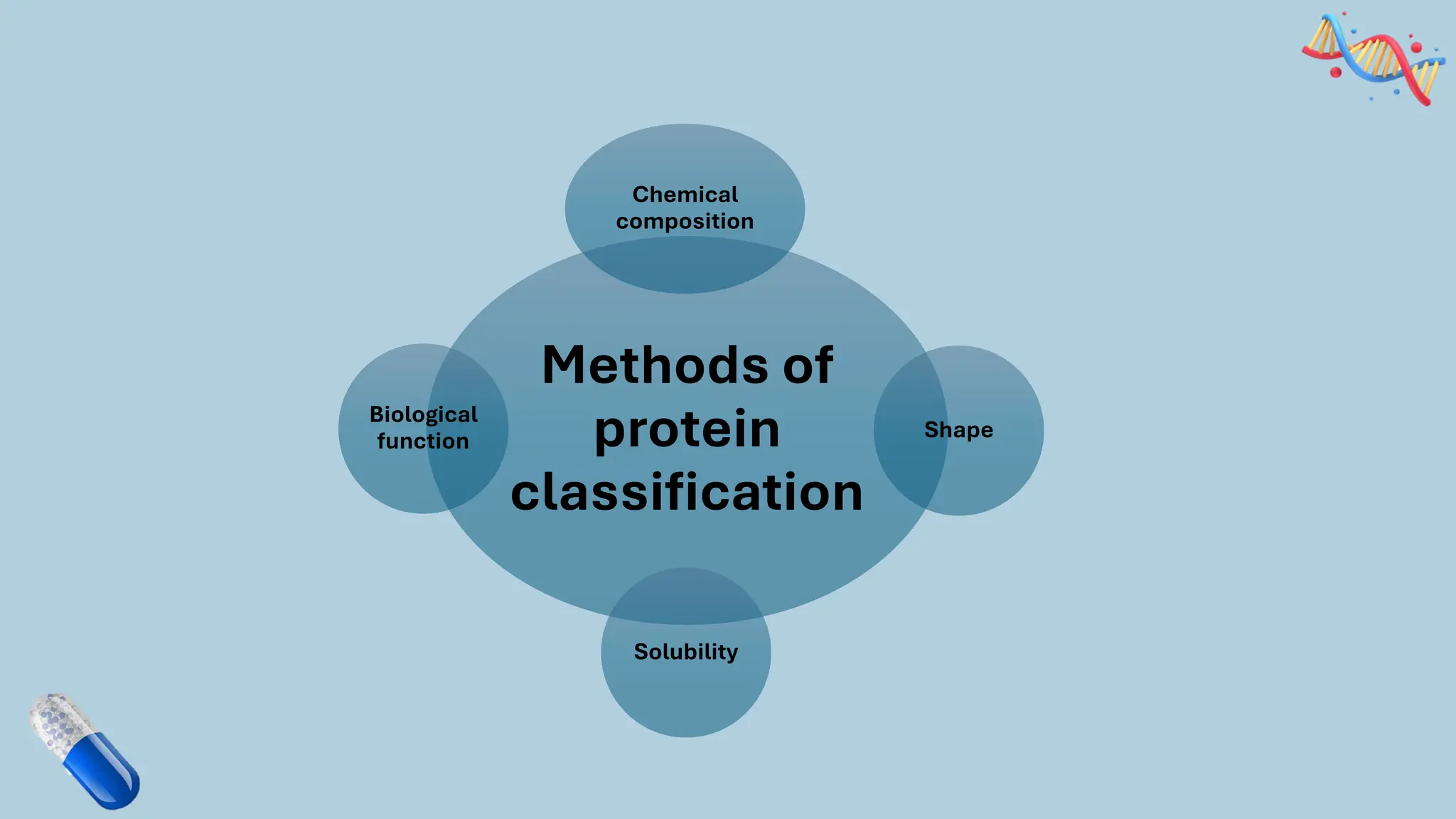
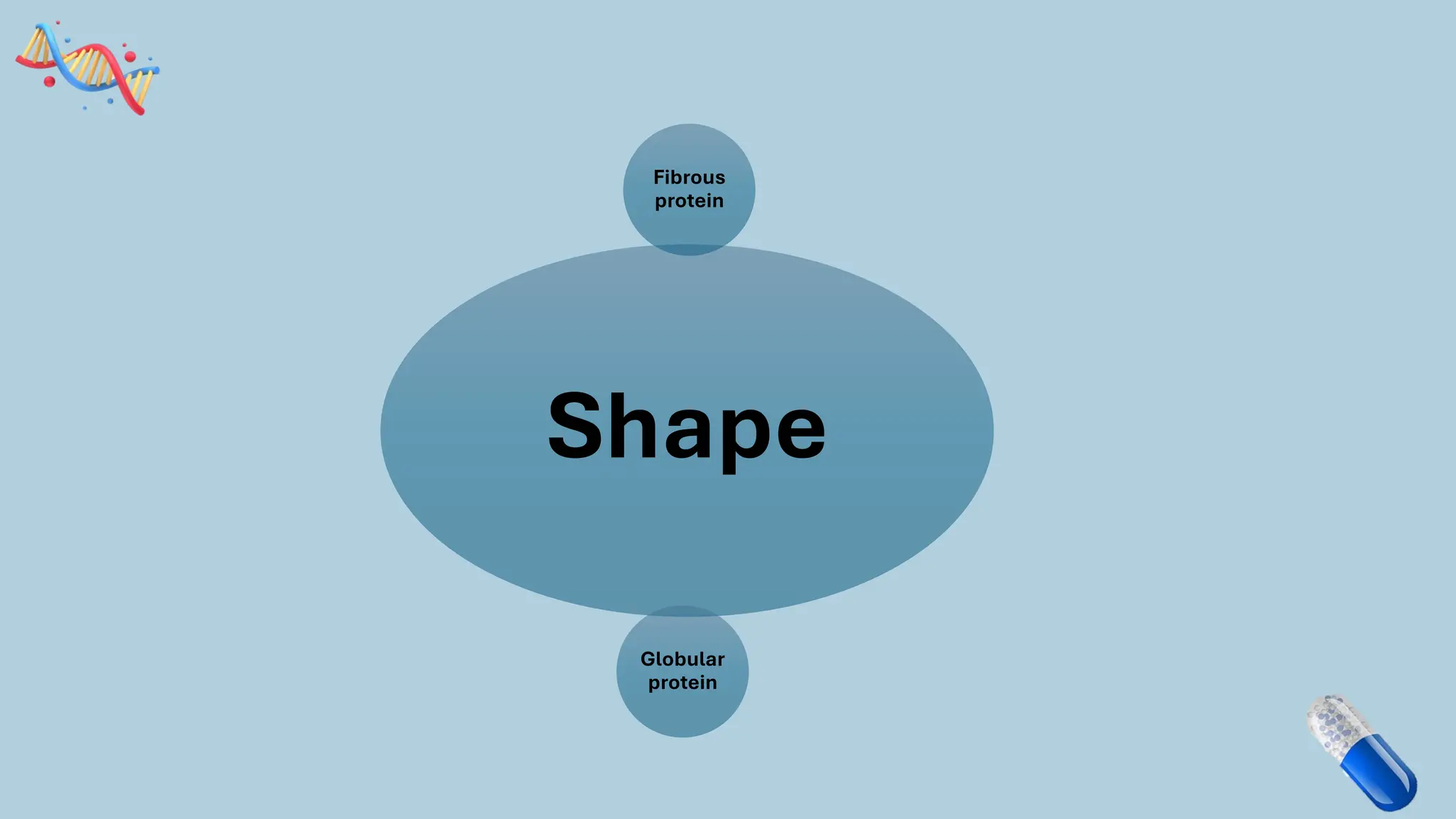
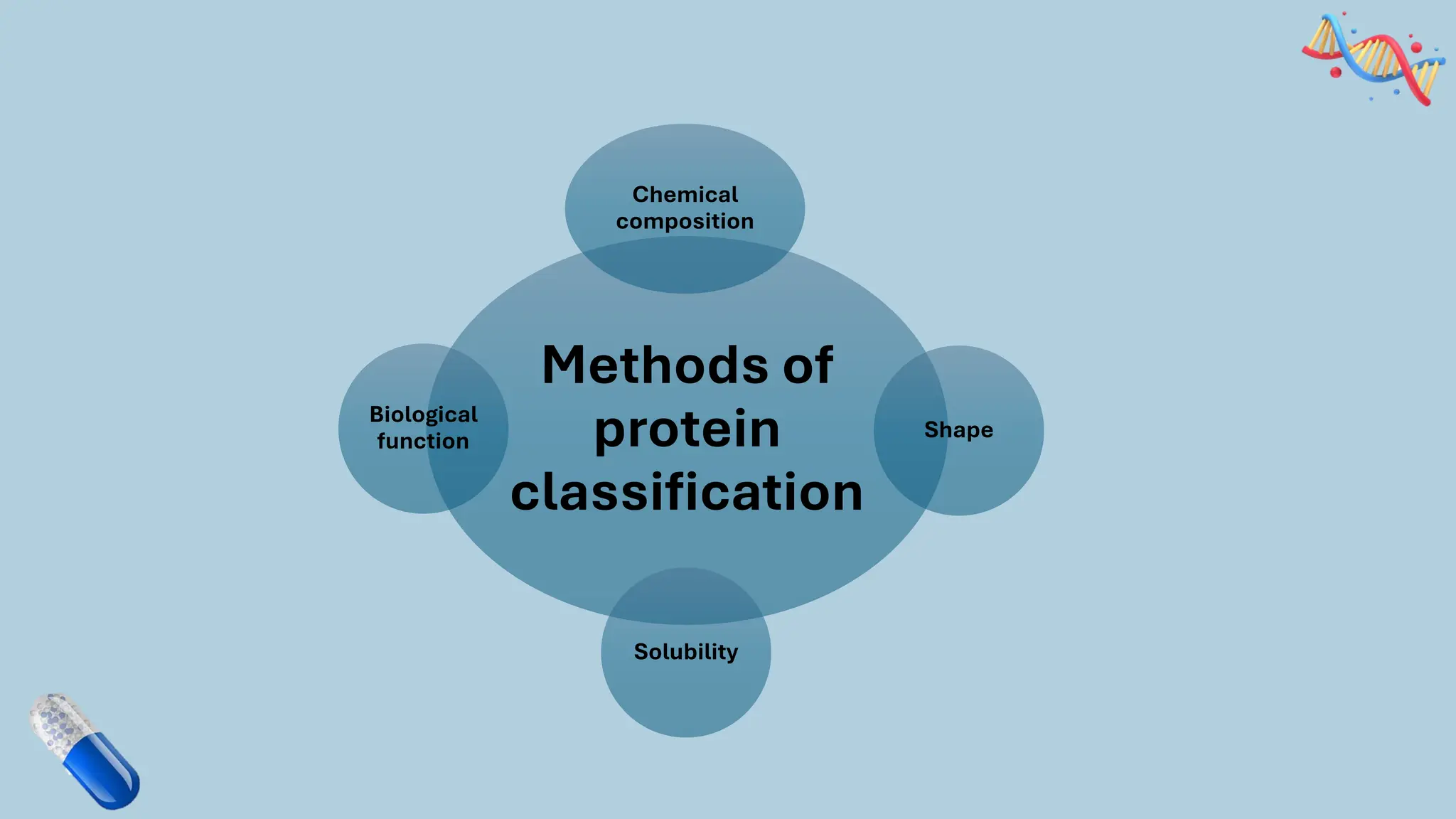
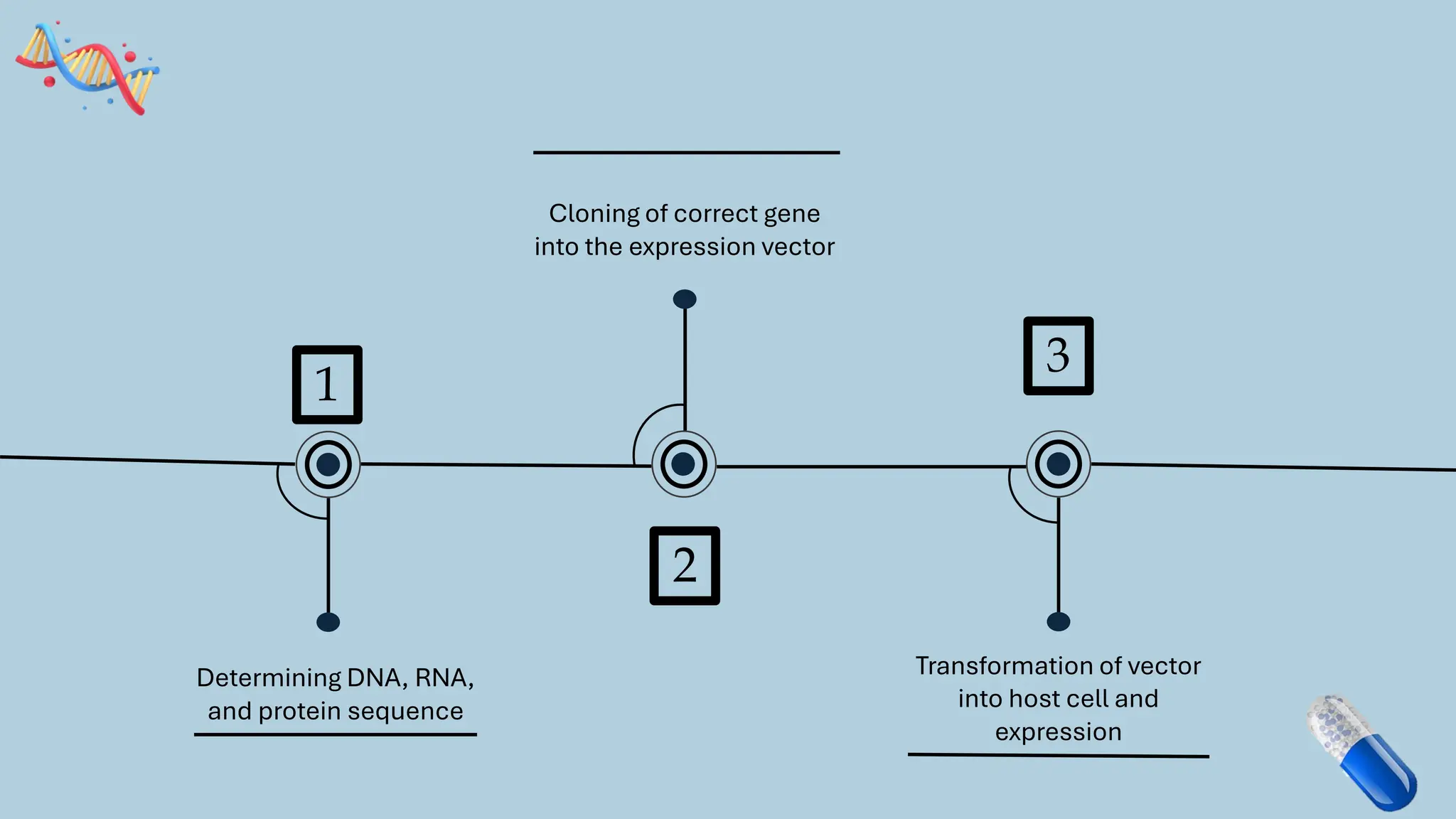
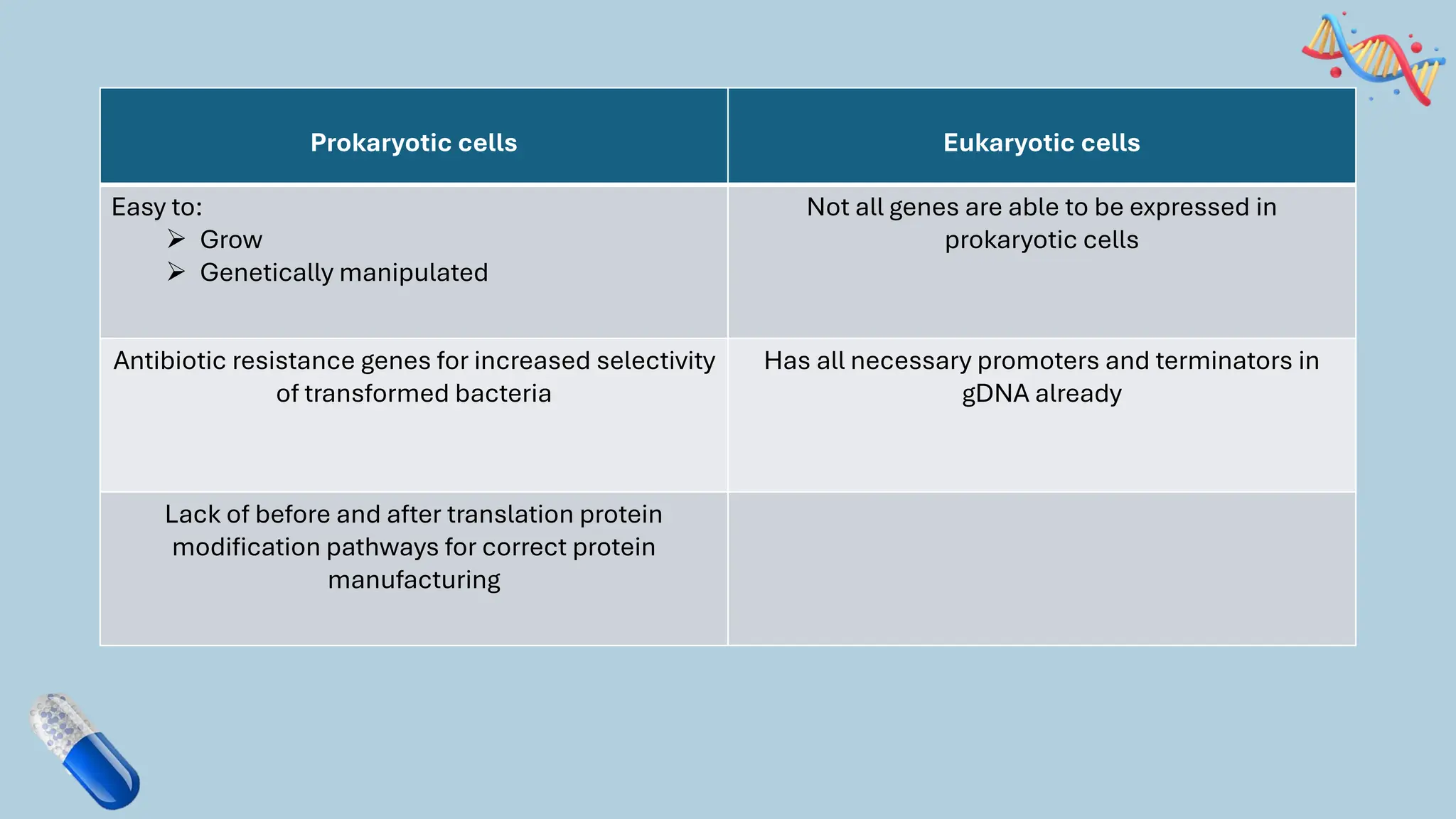
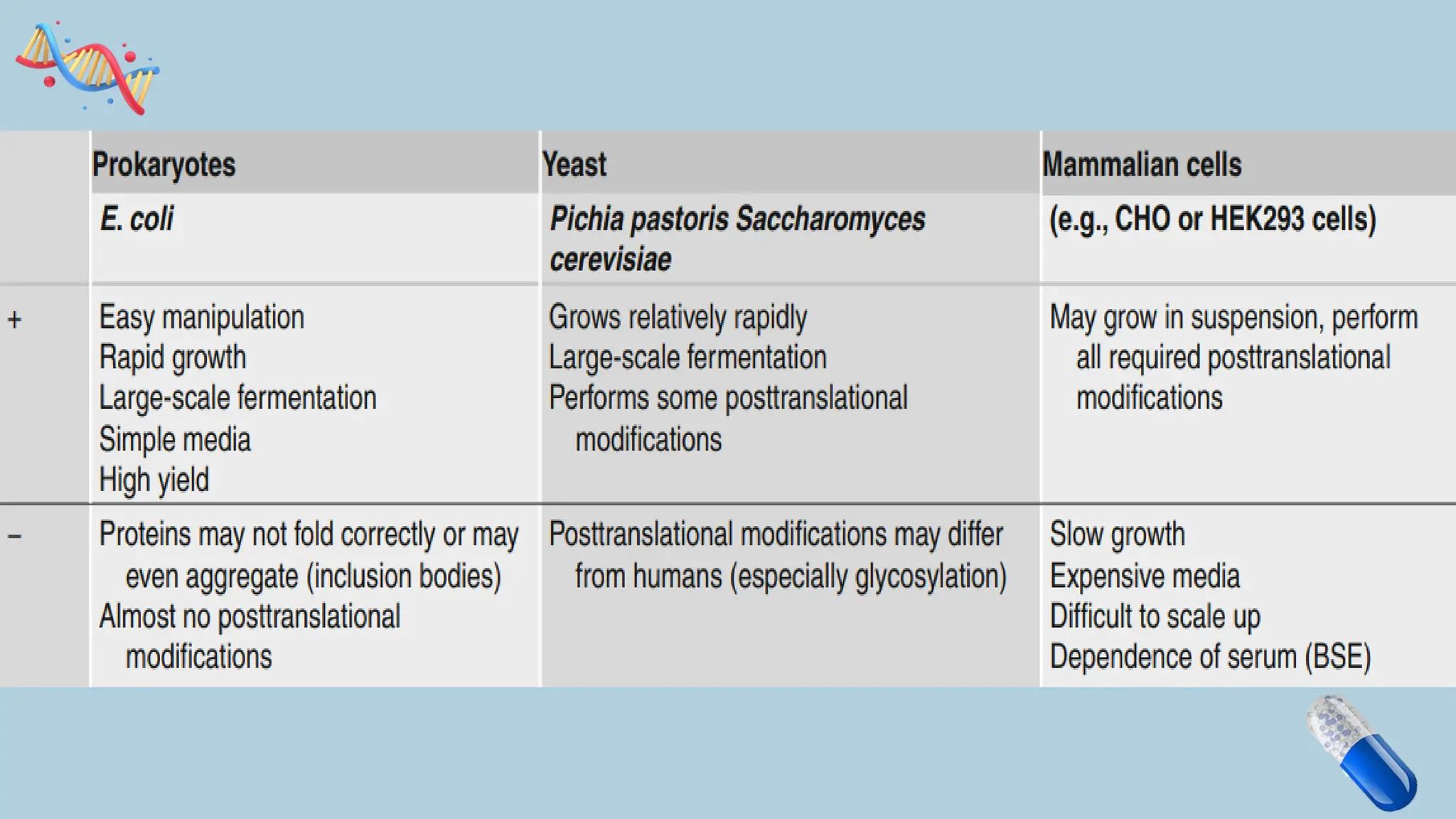
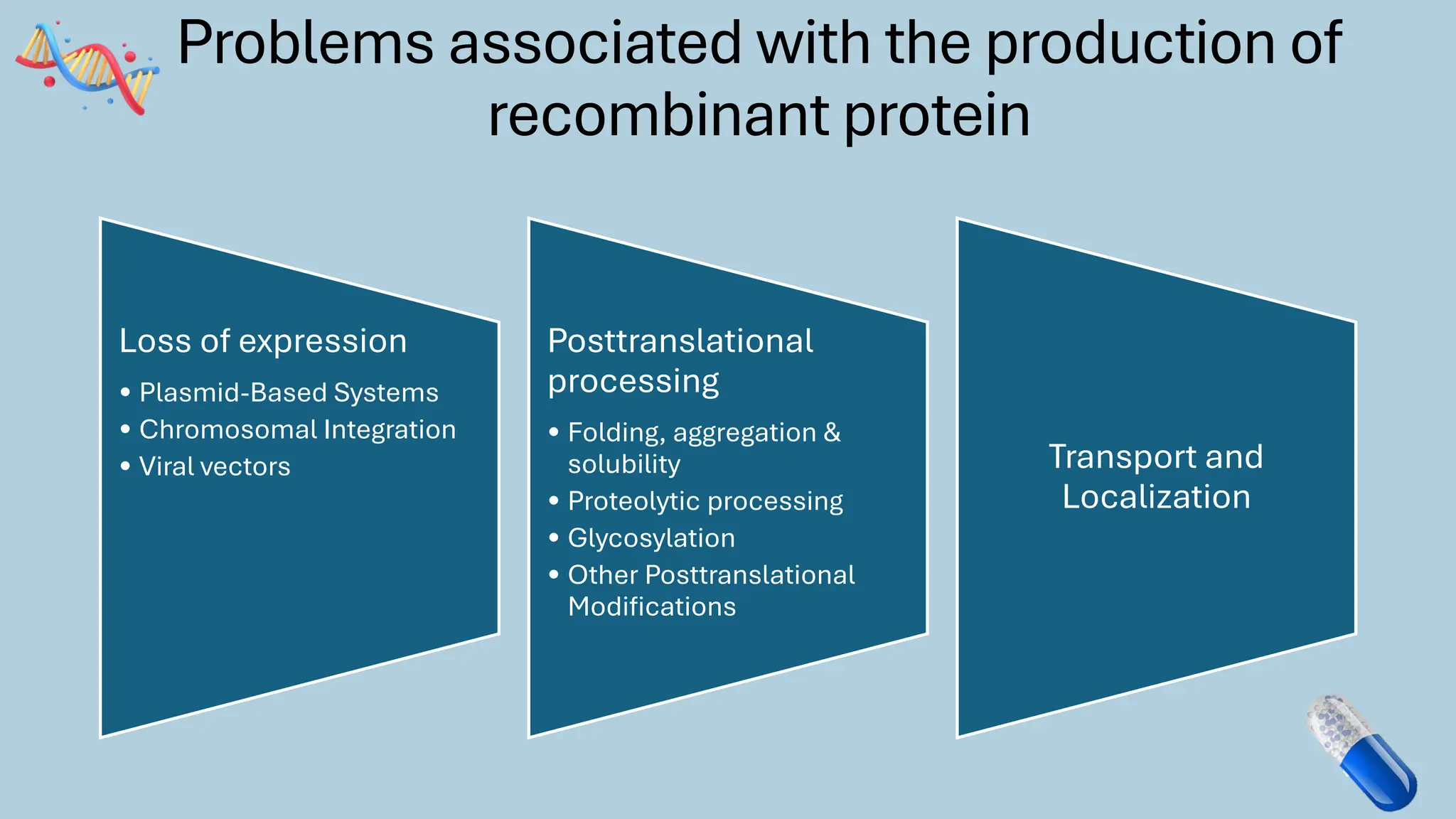
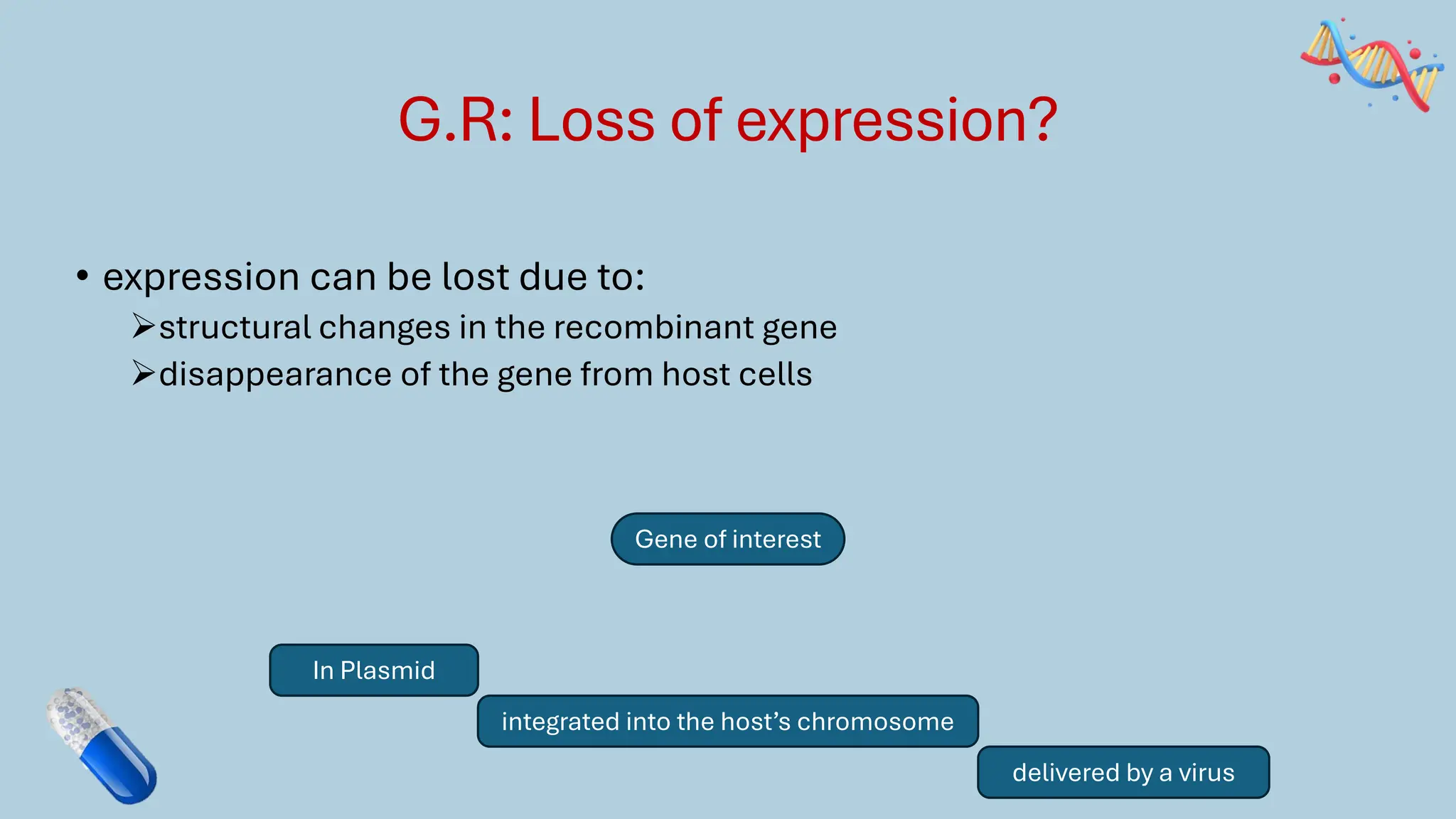
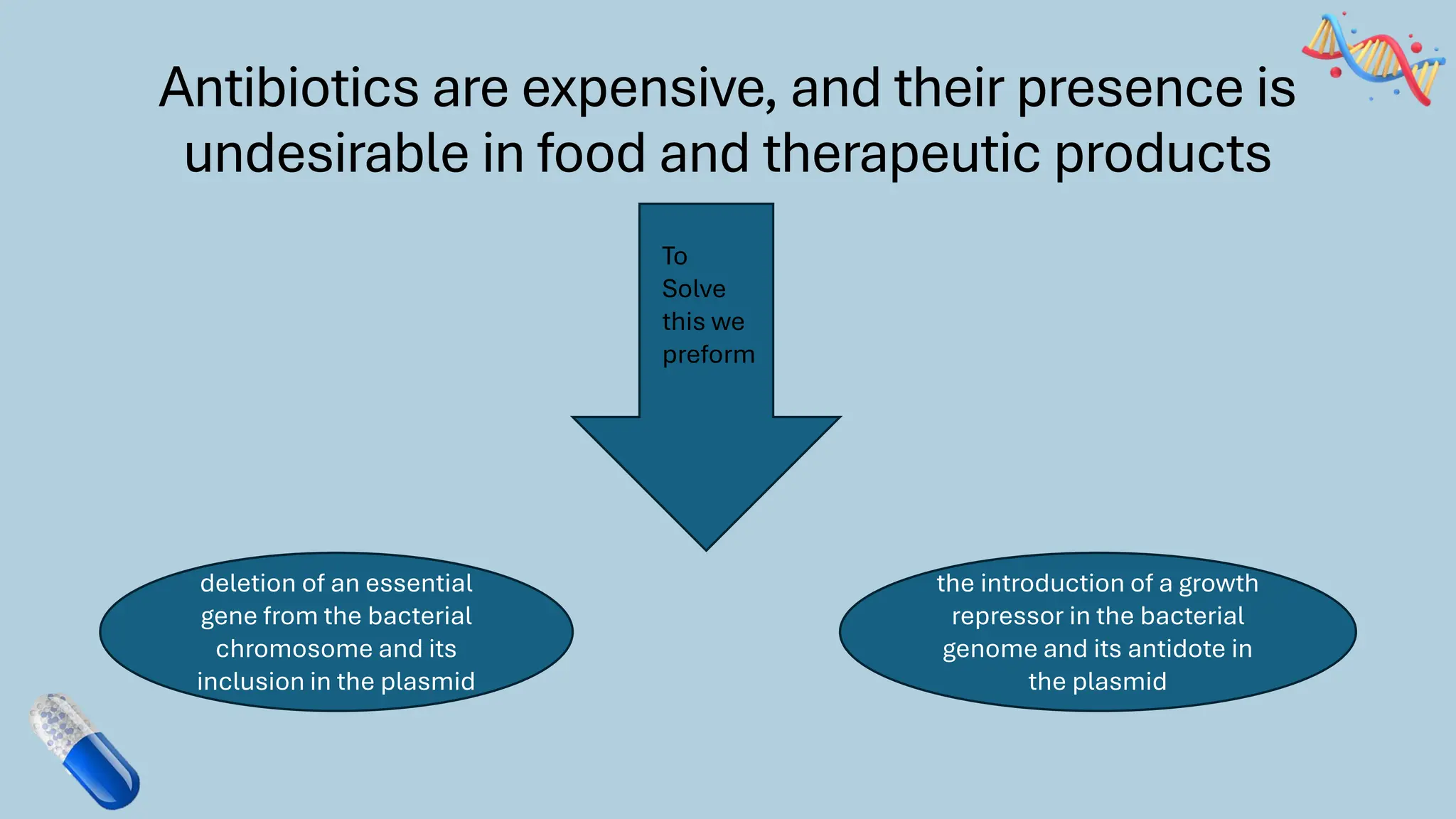
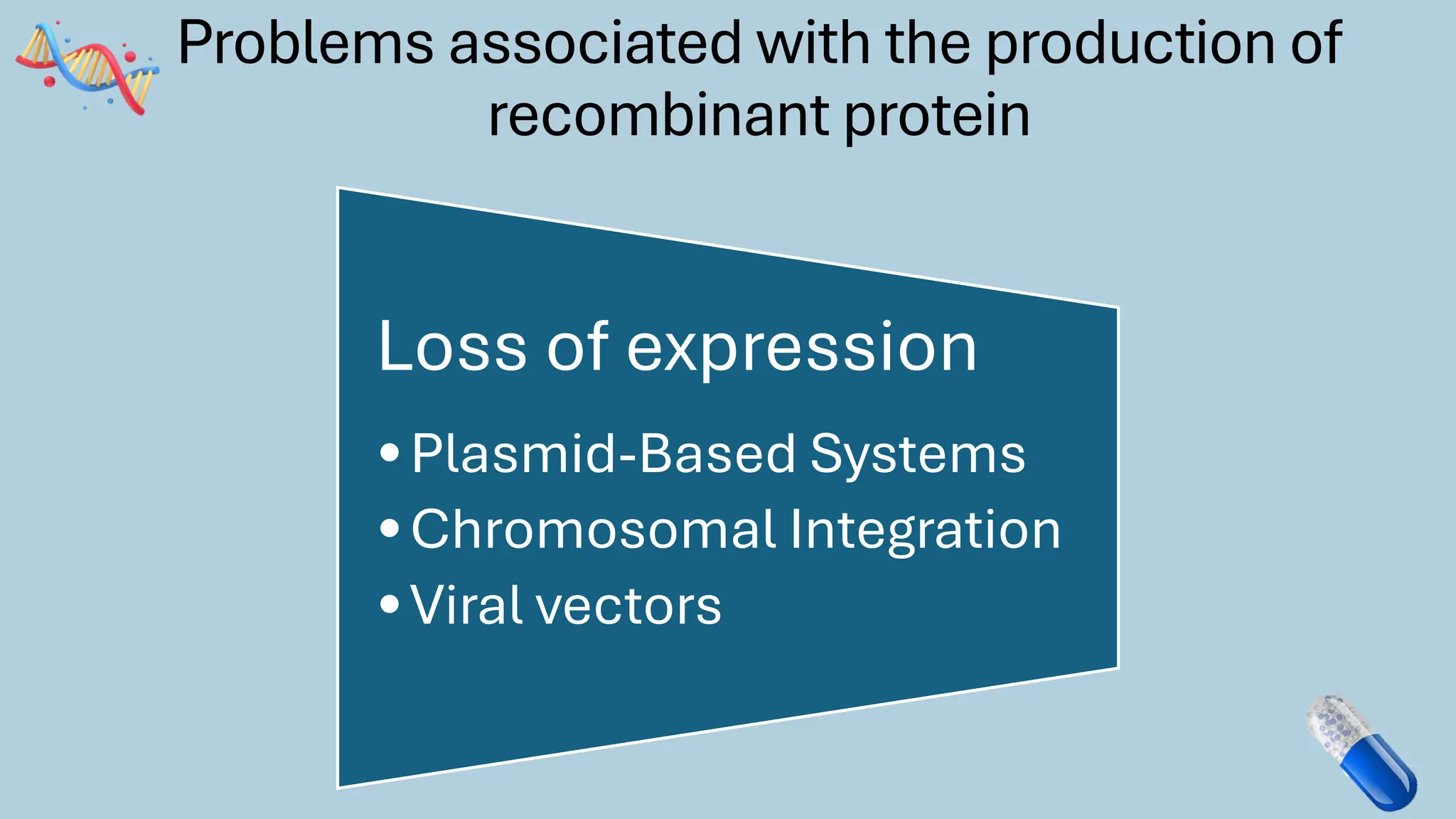
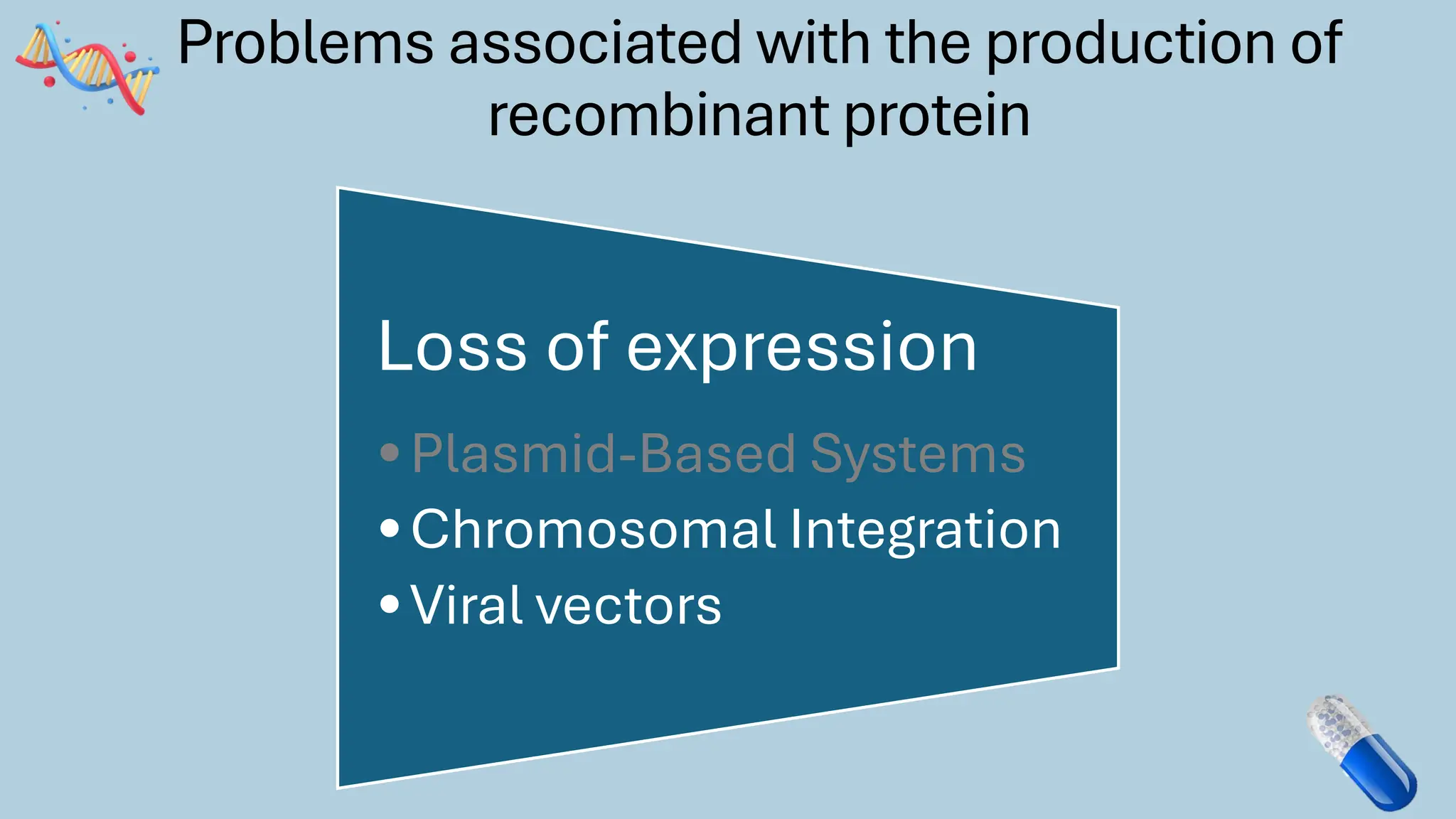
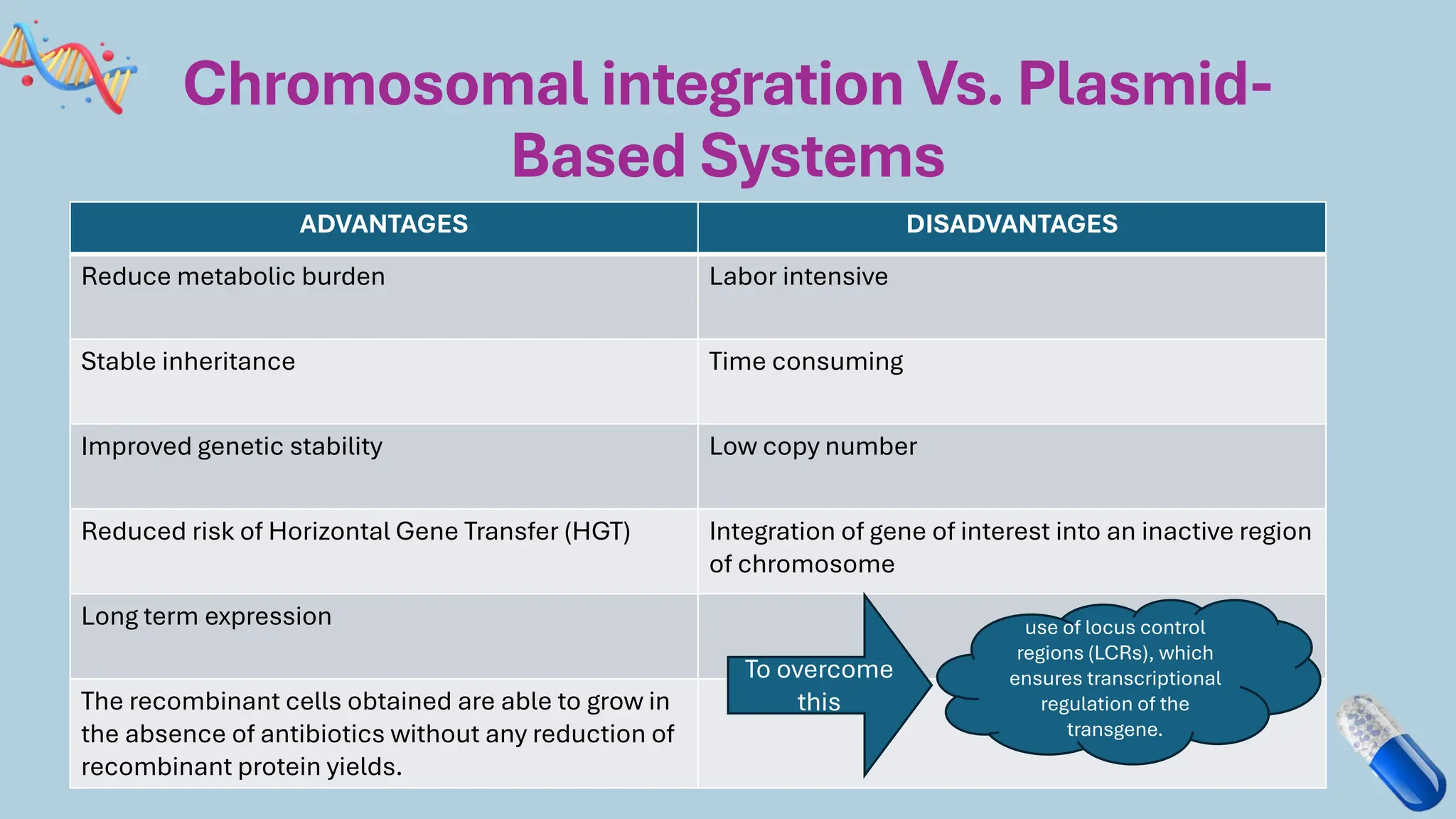
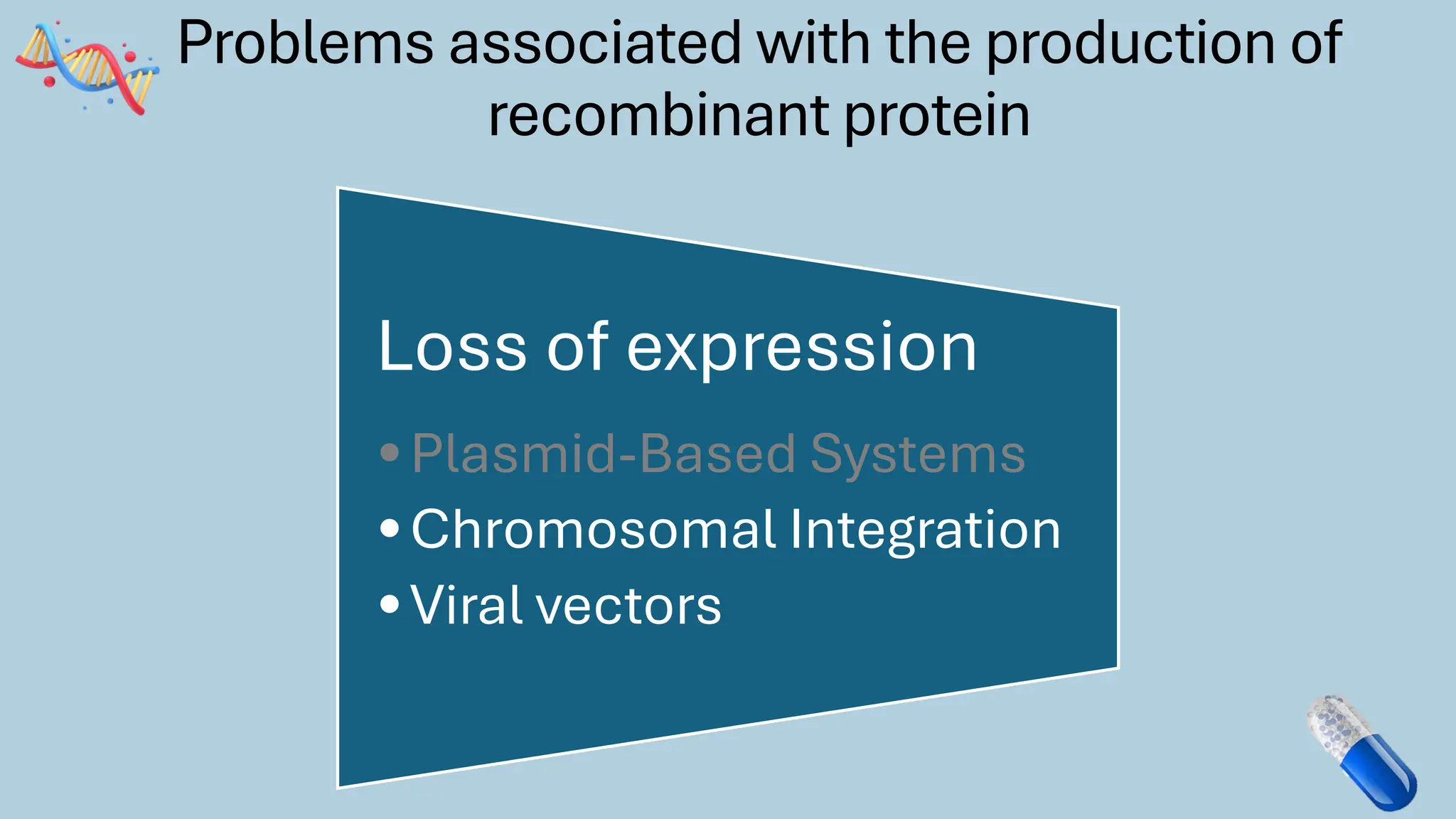
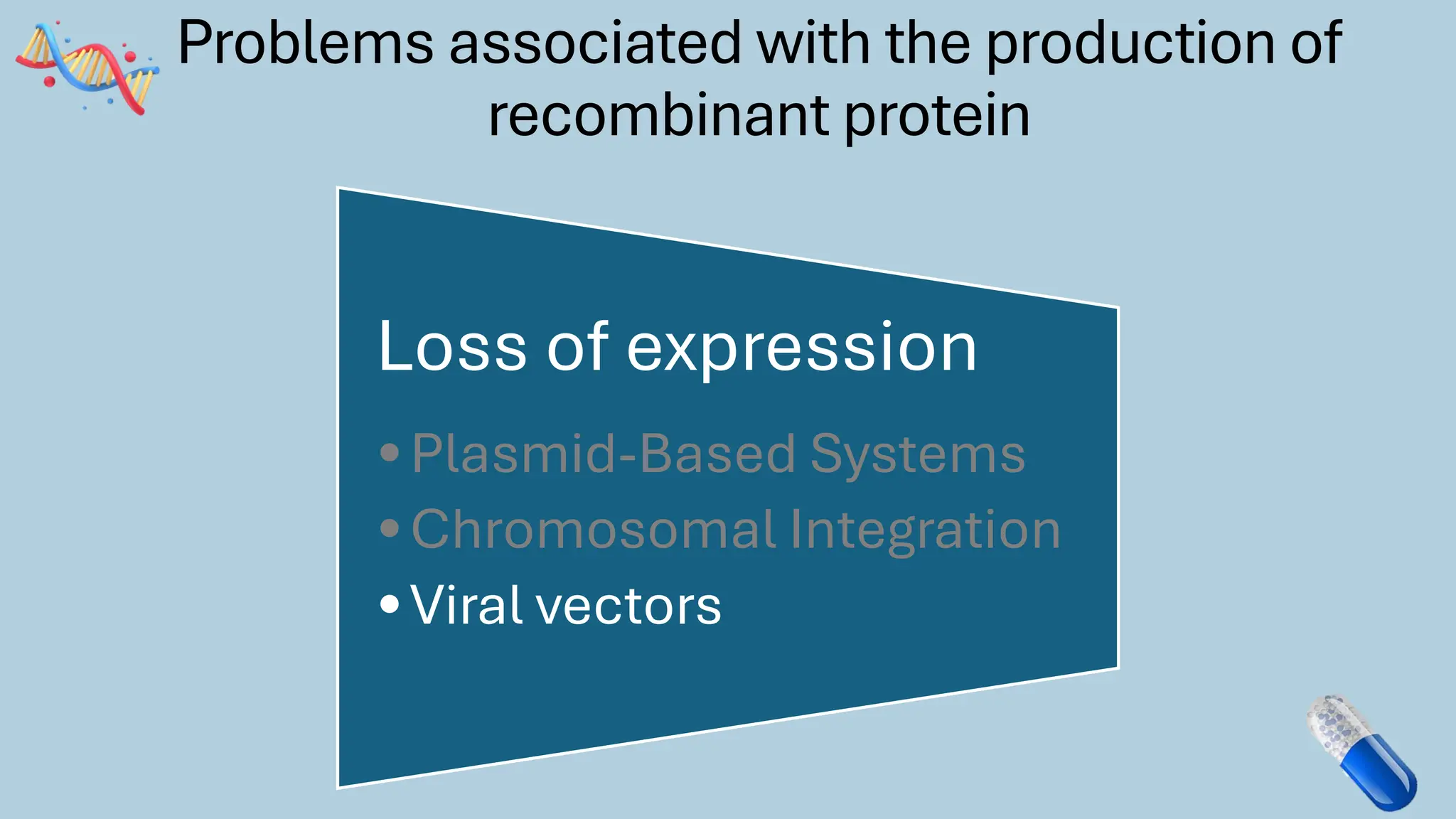
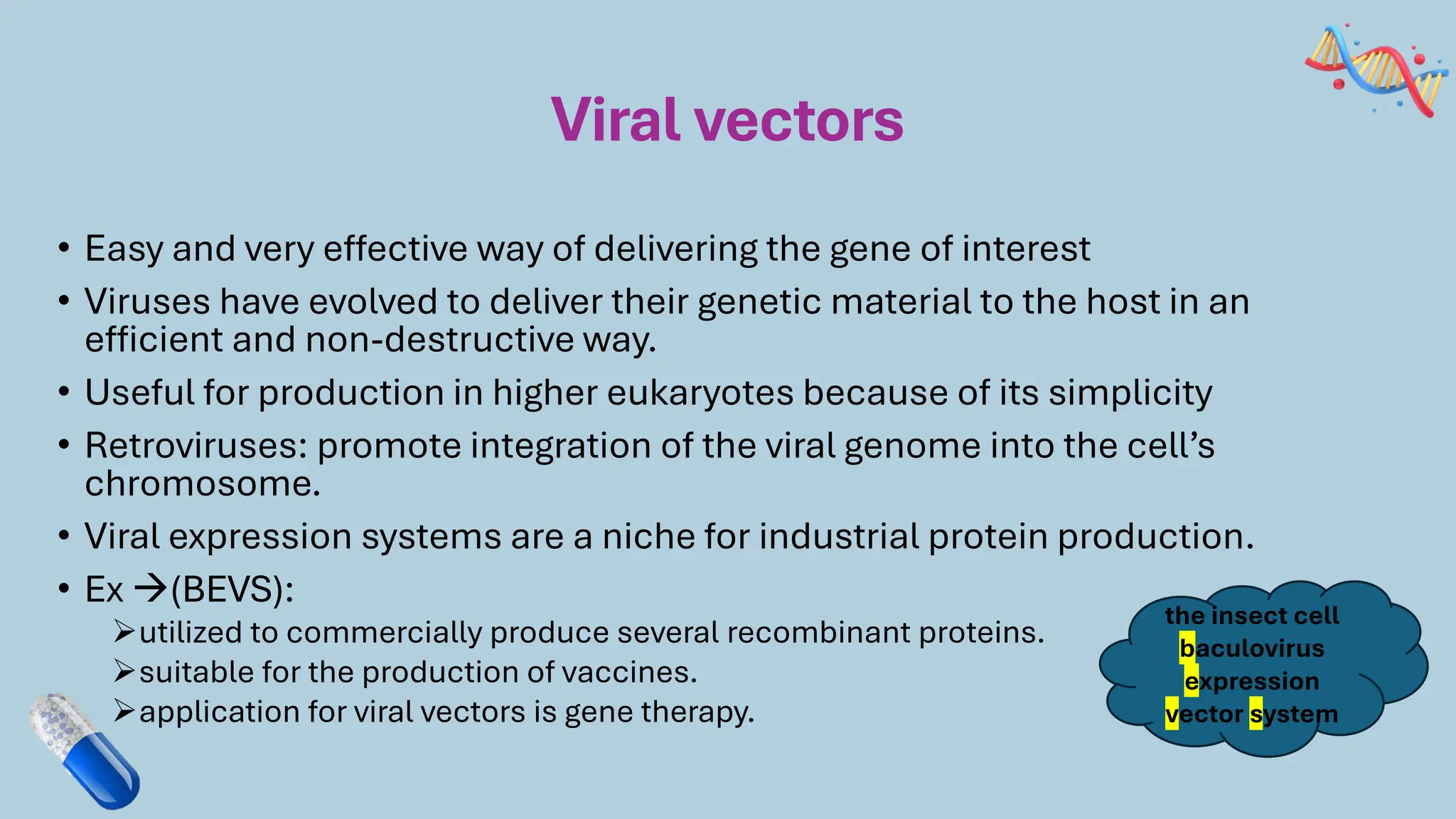
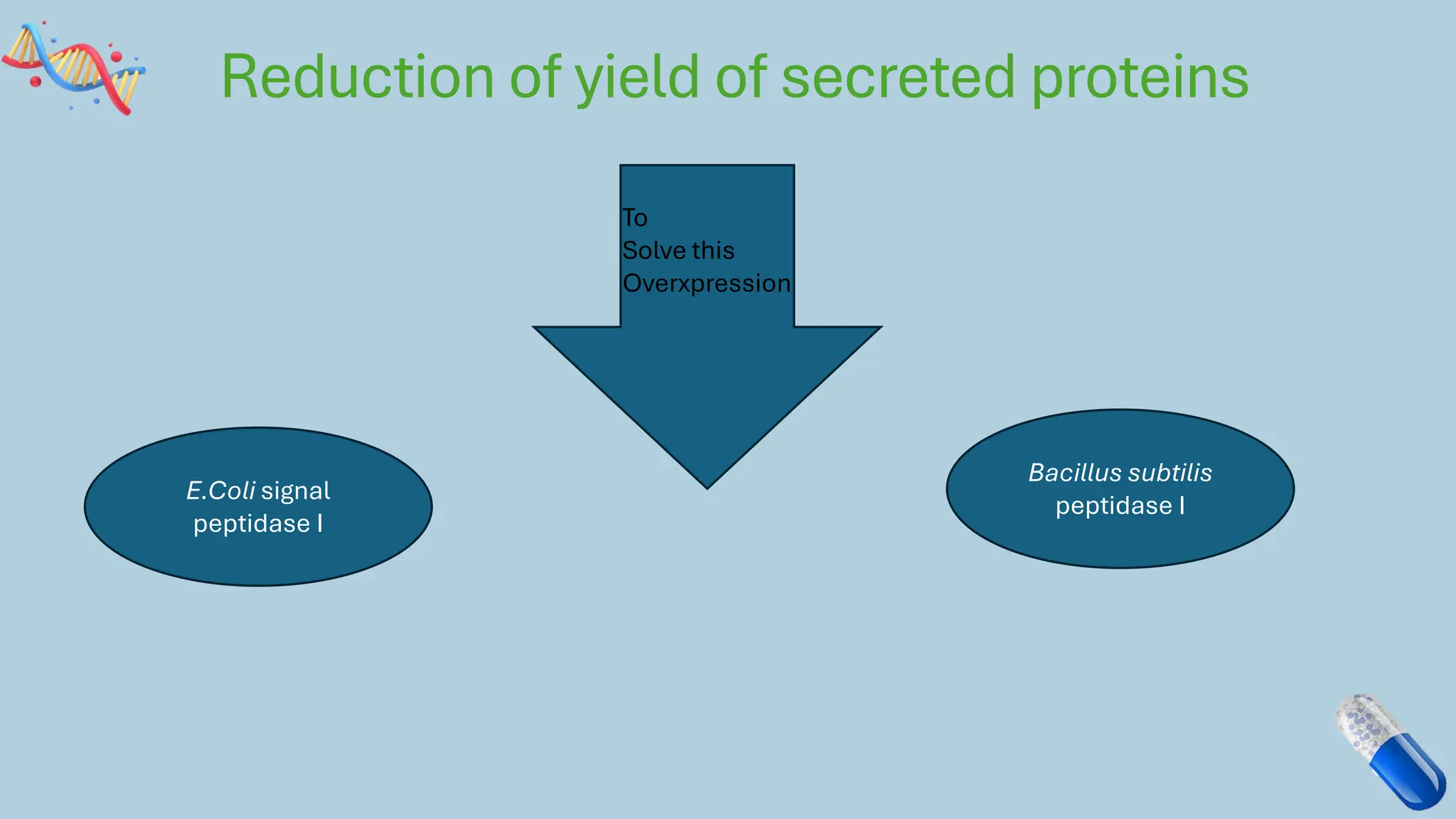
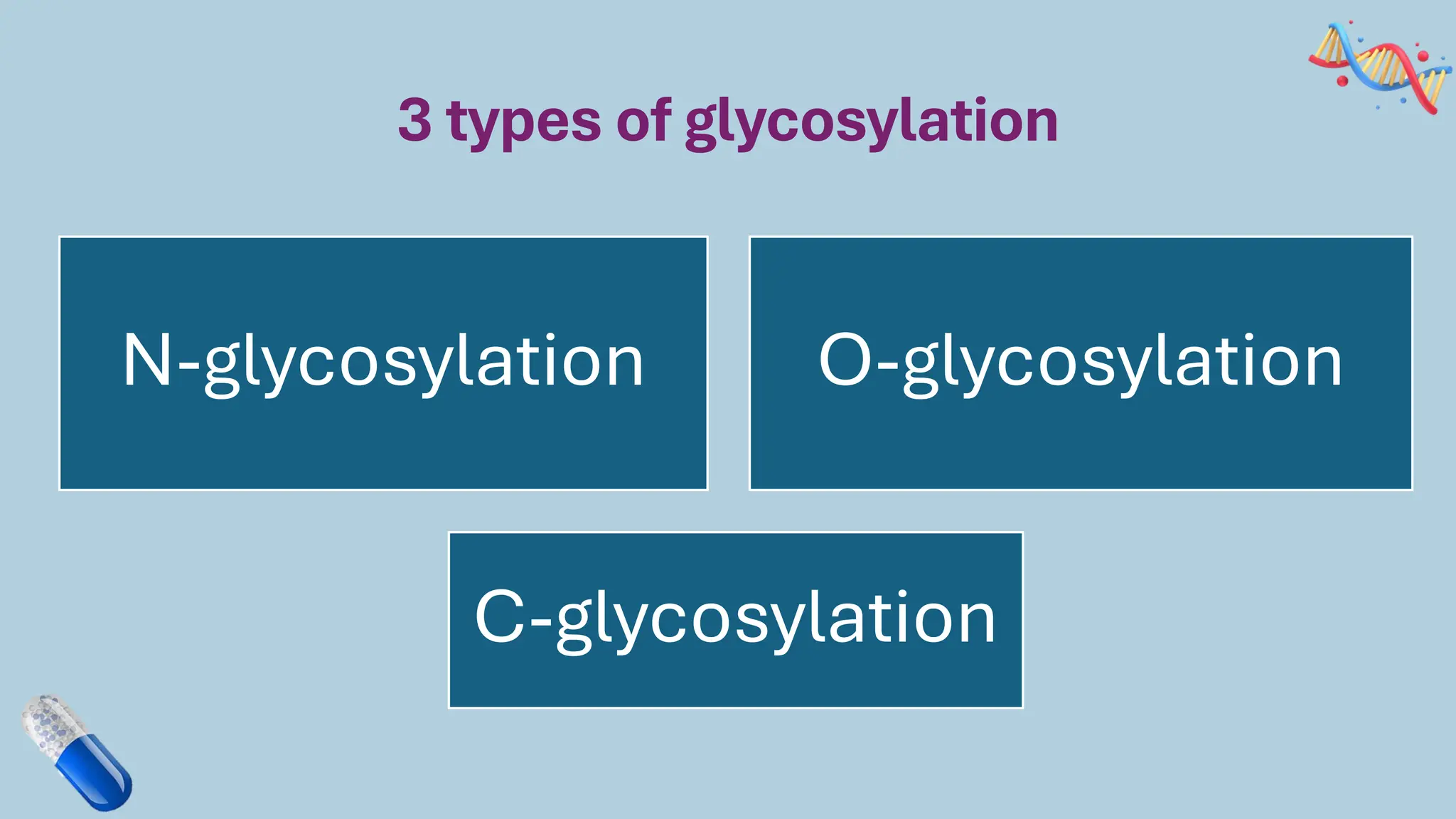
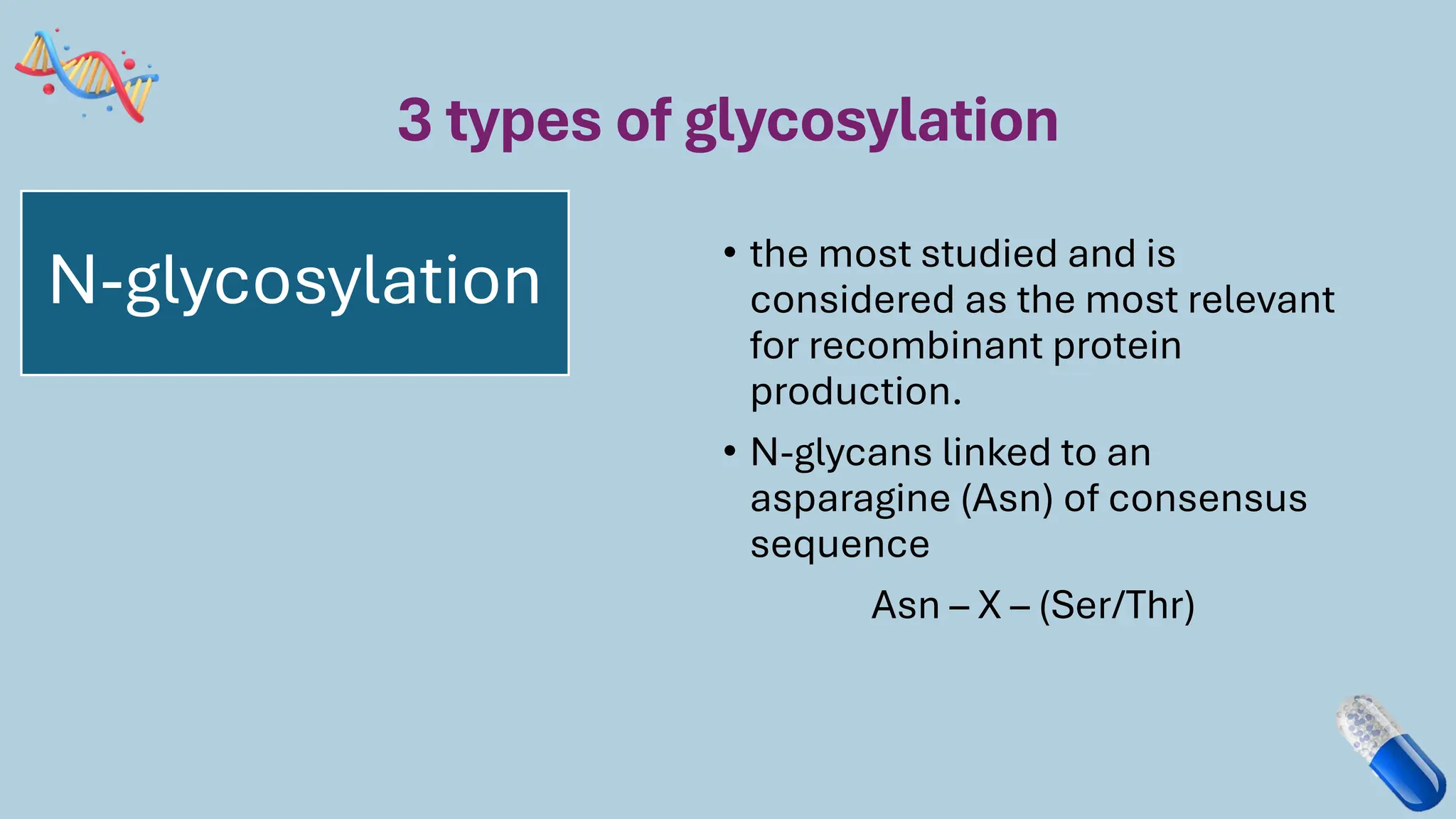
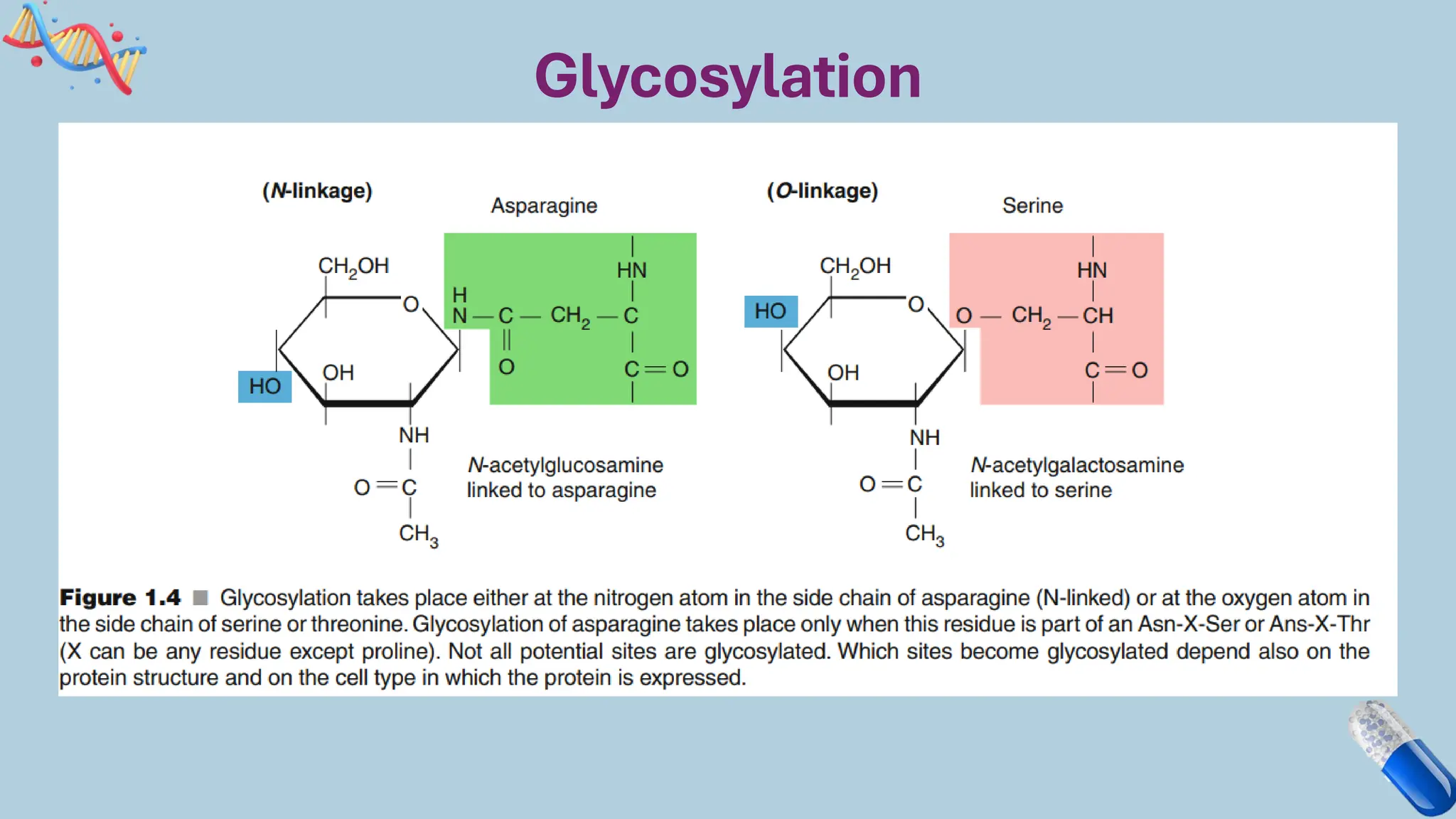
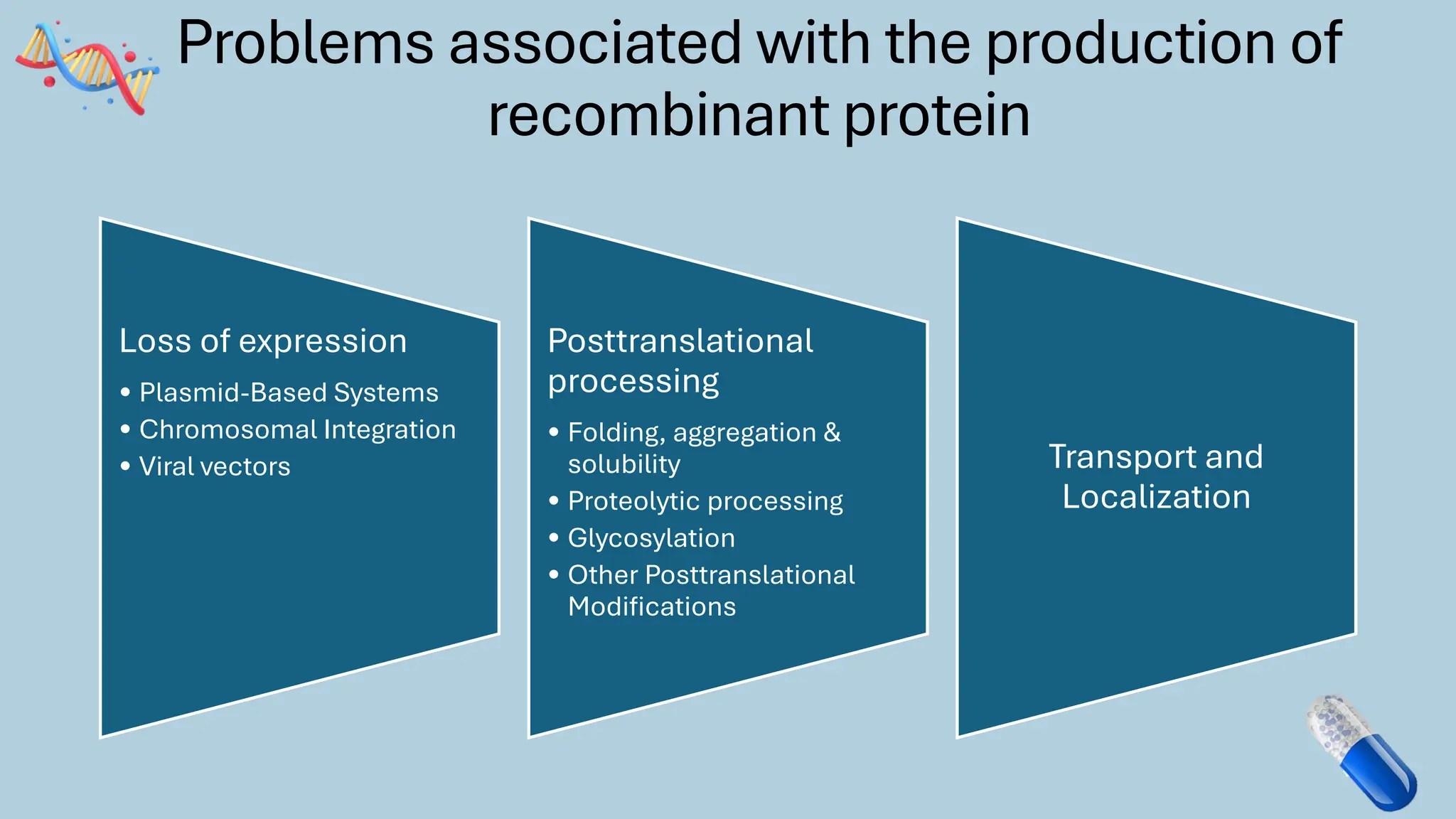
The document discusses the challenges and methods associated with the production of recombinant protein antibodies, highlighting issues related to gene expression stability in various systems (plasmid-based, chromosomal integration, viral vectors). It details posttranslational processing issues, such as protein folding, aggregation, and glycosylation, which affect protein functionality and yield. Various strategies, including engineering plasmids and the use of fusion proteins, are mentioned as potential solutions to enhance protein production and stability.