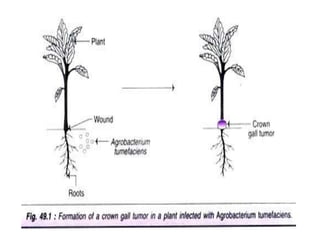
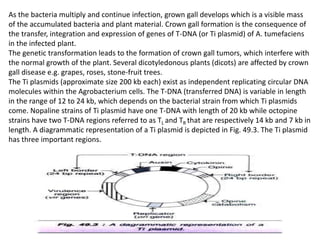
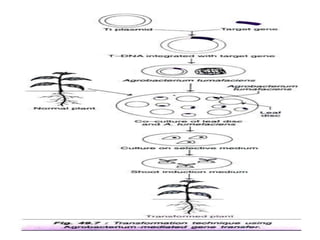
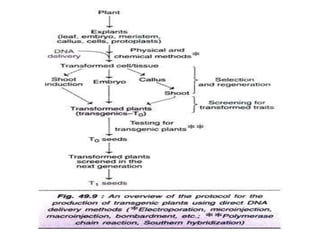
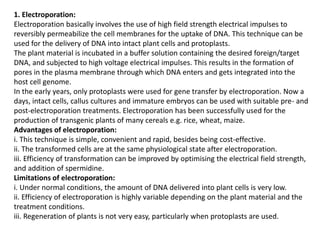
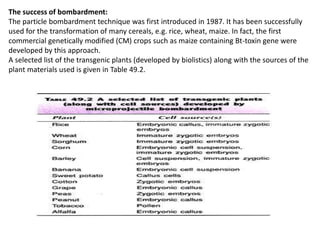
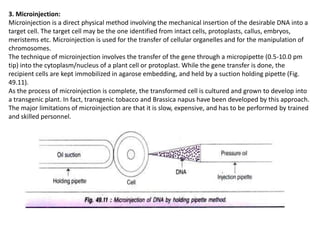
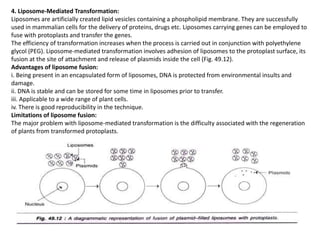
The document discusses gene transfer methods in plants, focusing on vector-mediated and direct DNA transfer techniques. Agrobacterium-mediated transformation is highlighted as the leading method due to its natural efficiency in plant genetic engineering, involving the transfer of tumor-inducing plasmids that integrate into the host genome. It also covers the limitations and advantages of various direct DNA transfer methods, including electroporation, particle bombardment, and microinjection, along with their respective effectiveness and challenges.