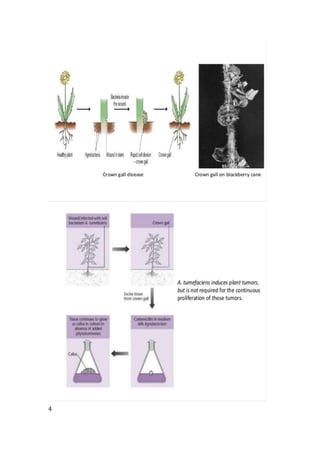
1. The document discusses Agrobacterium-mediated plant transformation using the soil bacteria Agrobacterium tumefaciens and Agrobacterium rhizogenes.
2. It describes the Ti and Ri plasmids contained in A. tumefaciens and A. rhizogenes that allow for transfer of T-DNA containing genes into plant cells.
3. The binary vector strategy is discussed as an effective method for inserting foreign genes into the T-DNA and transforming plant cells.