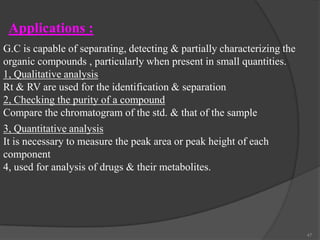
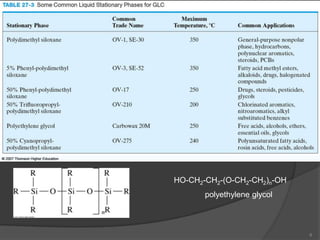
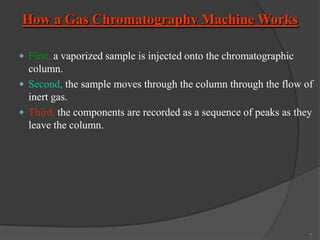
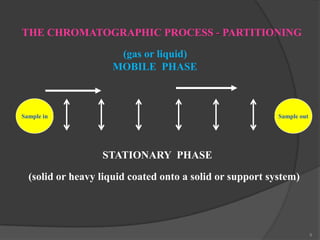
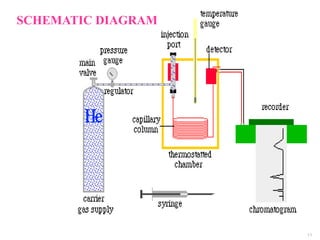
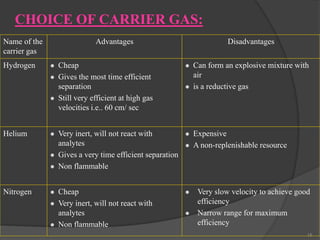
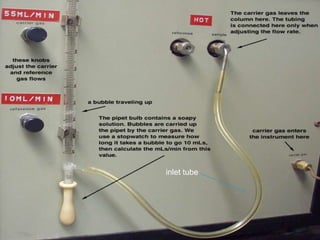
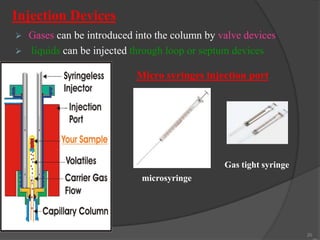
This document provides an overview of gas chromatography. It begins with an introduction to chromatography and lists some common chromatographic techniques. It then describes the basic components and working of gas chromatography, including the carrier gas, columns, temperature control, detectors, and how the chromatographic process separates components based on partitioning between a mobile and stationary phase. The principle of gas chromatography is described as partition, with examples of different types of columns and factors that influence chromatographic separation. The key components of a gas chromatography system and their functions are also summarized.



![Gas chromatography
In Gas chromatography, the components of a
vapouraised sample are fractionated as a consequence of a
partition between a mobile gaseous phase and a stationary
phase held in a column.
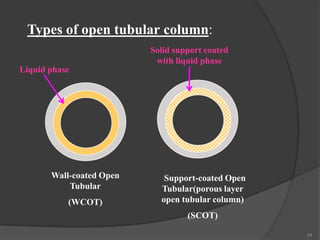
According to the nature of stationary phase, Gas
chromatography may be
(a). Gas solid chromatography [GSC]
(b). Gas liquid chromatography [GLC]
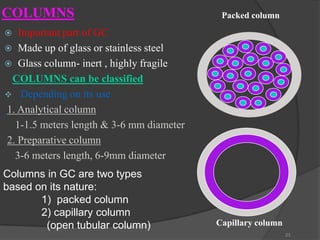
Carrier gas
column
detector
Basic chromatographic arrangement
4](https://image.slidesharecdn.com/nikhiseminor1-140208213037-phpapp02/85/GAS-CHROMATOGRAPHY-4-320.jpg)


















![ii. Trifluoroacetic anhydride (TFAA) is one common reagent used for
acylation.
NH
+
O
COCF3
N-CO-CF3 + HOCOCF
3
COCF3
methamphetamine
Drug-of-abuse confirmation testing by GC
iii. Another set of reagents used for solute with primary and secondary
amines, as well as hydroxyl and thiol groups are N-Methylbis[trifluoroacetamide] (MBTFA). The reaction is under mild nonacidic
conditions.
H
Me
CF3
N
Byproduct is volatile
O
45](https://image.slidesharecdn.com/nikhiseminor1-140208213037-phpapp02/85/GAS-CHROMATOGRAPHY-45-320.jpg)