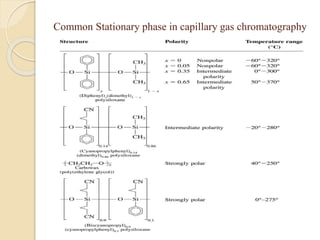
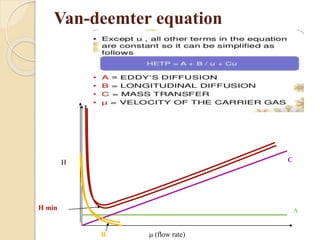
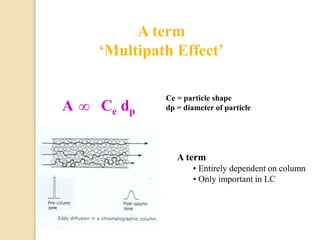
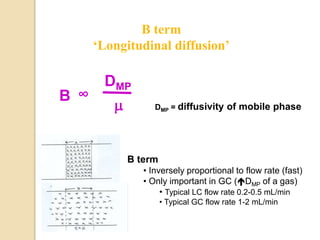
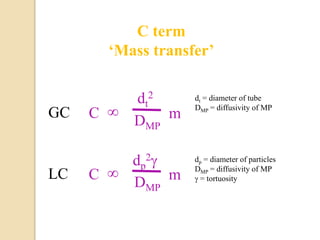
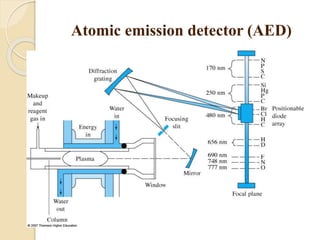
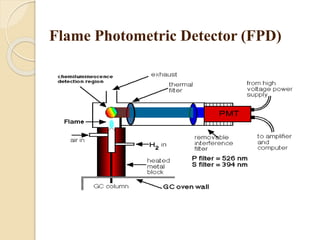
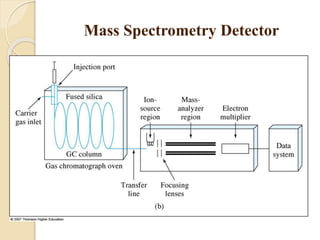
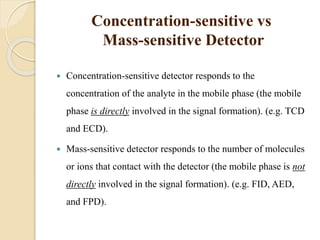
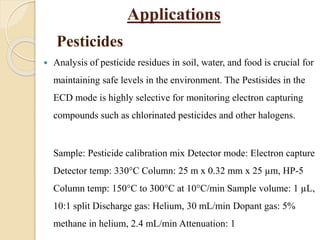
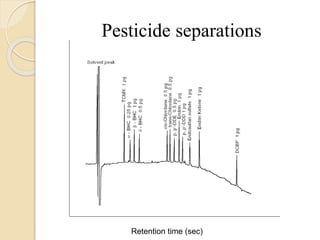
1. Gas chromatography (GC) is a popular method for separating and analyzing compounds due to its high resolution, low detection limits, speed, accuracy, and reproducibility.
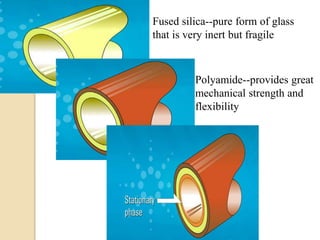
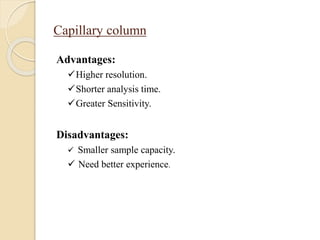
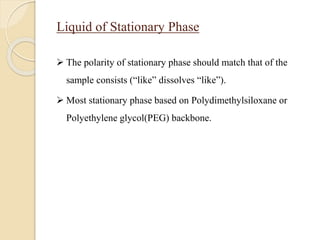
2. GC works by separating compounds based on differences in their partitioning behavior between a mobile gas phase and stationary phase in the column.
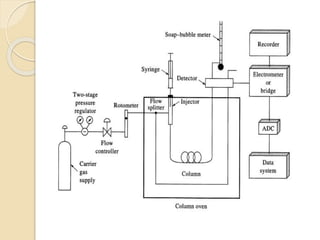
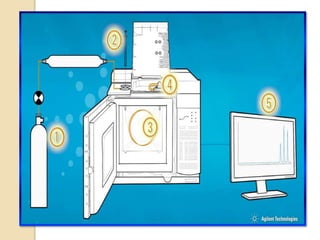
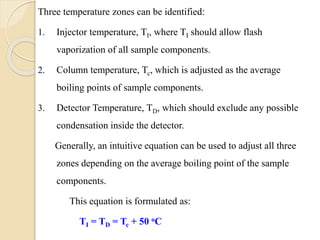
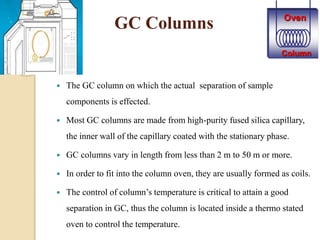
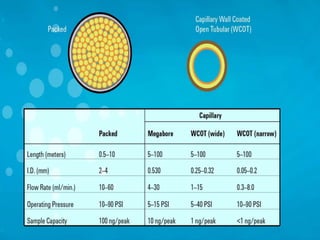
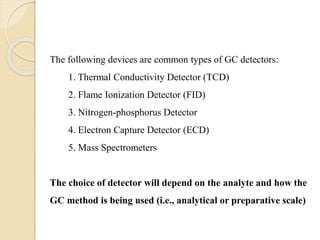
3. A basic GC system consists of a gas source, injector or sample inlet, chromatographic column inside an oven for temperature control, detector, and computer or recorder to analyze the separated compounds.