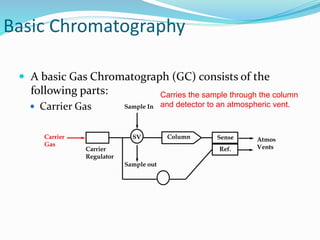
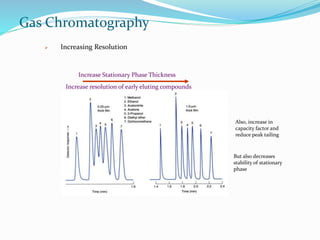
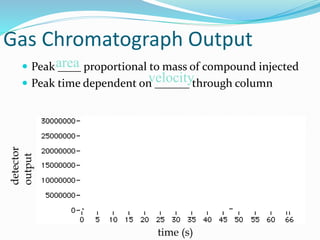
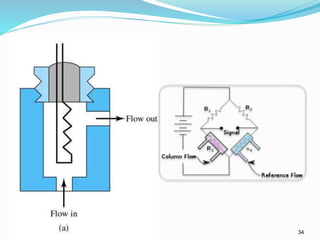
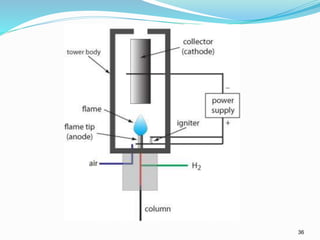
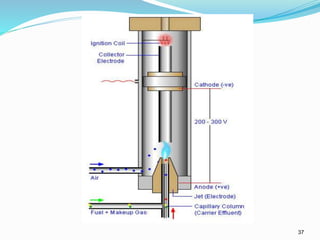
This document provides an overview of gas chromatography. It describes the basic components and process of gas chromatography including the carrier gas, sample injection system, columns, temperature and pressure programming, and common detectors like the thermal conductivity detector and flame ionization detector. The goal of gas chromatography is to separate a mixture into individual components using a mobile gas phase and stationary column packing material over time based on differences in how components partition between the two phases.