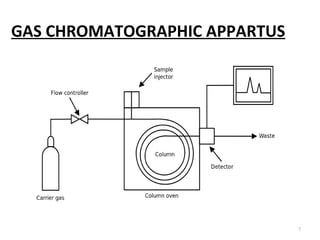
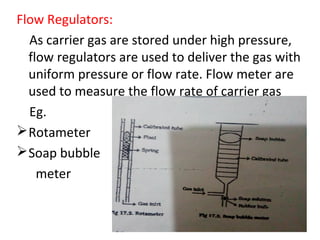
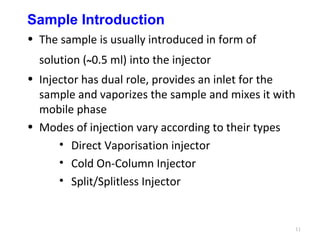
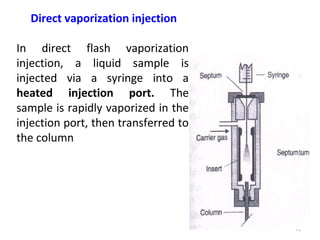
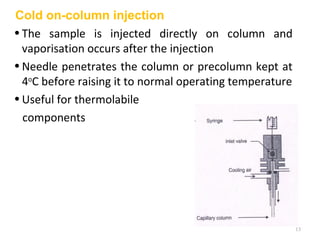
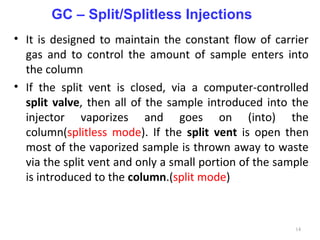
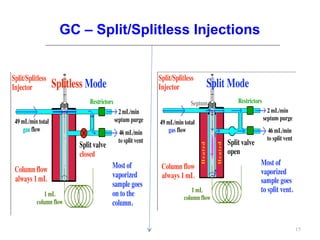
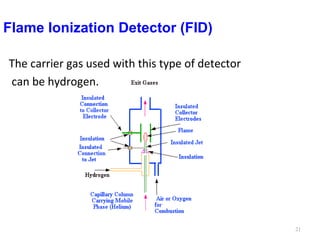
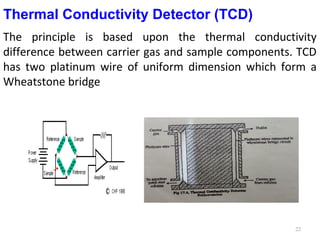
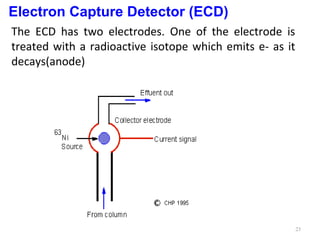
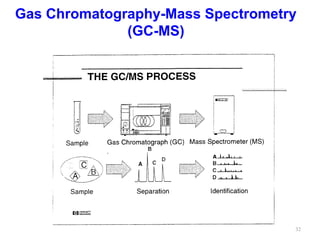
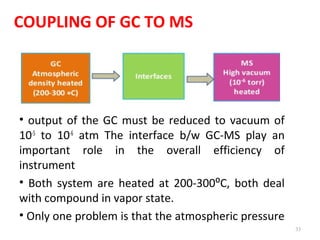
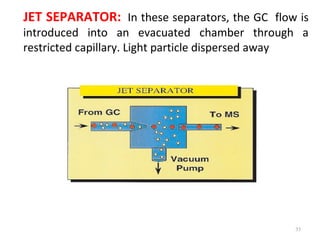
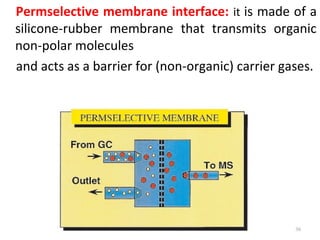
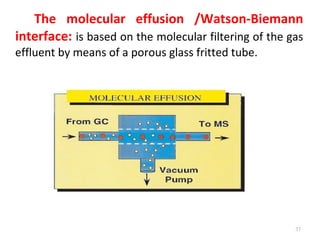
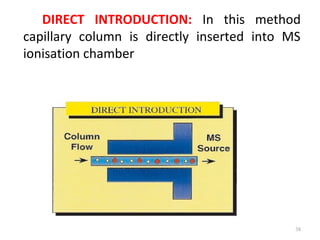
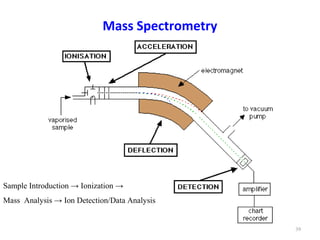
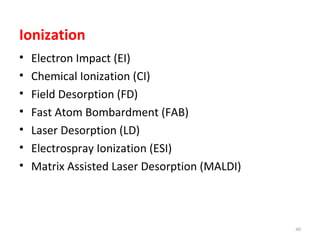
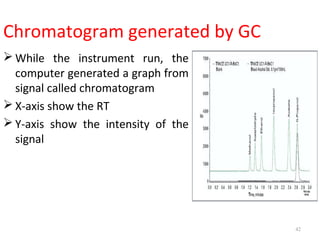
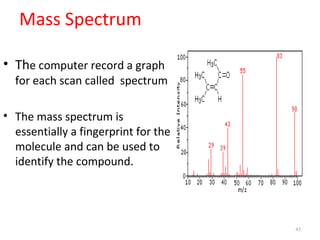
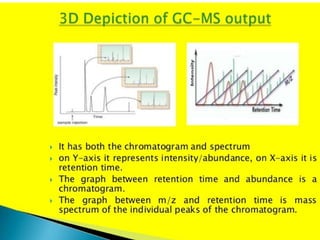
Gas chromatography-mass spectrometry (GC-MS) combines the separation capabilities of gas chromatography with the mass analysis capabilities of mass spectrometry. It allows unknown substances to be separated, quantified, and identified. The document discusses the principles and components of GC and GC-MS, including sample introduction, columns, detectors, interfaces between GC and MS, ionization methods in MS, and interpretation of chromatograms and spectra. GC separates components which are then analyzed by MS to produce a 3D graph allowing identification of each separated component.