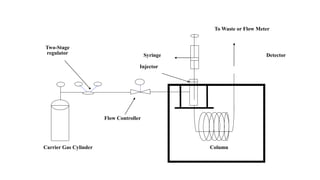
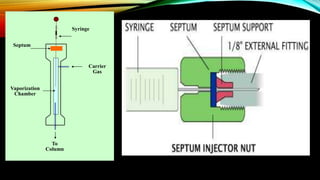
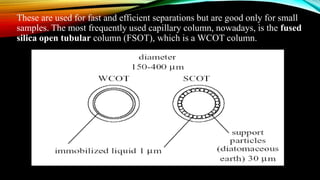
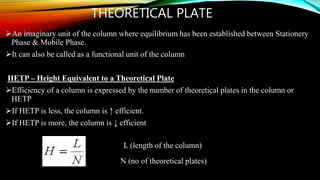
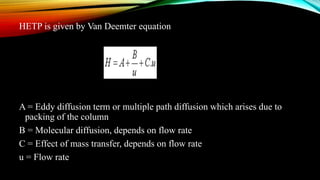
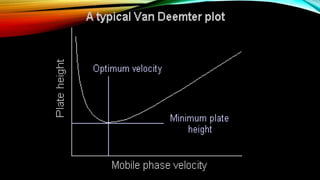
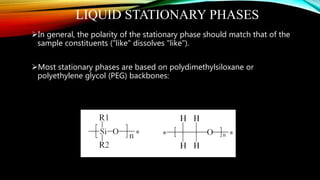
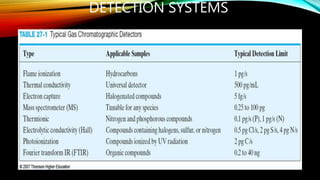
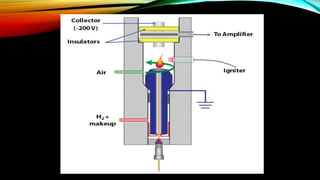
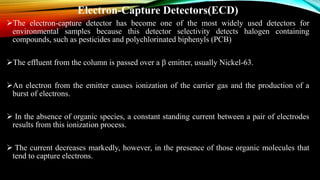
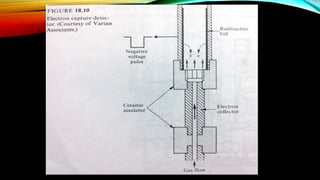
The document details gas chromatography (GC), a technique for separating and analyzing gaseous and volatile substances, which operates on principles of gas-solid (GSC) and gas-liquid chromatography (GLC). It covers the fundamental components, such as carrier gases, flow regulators, injectors, columns, and various detectors including Thermal Conductivity Detectors (TCD) and Flame Ionization Detectors (FID), highlighting their advantages and application areas. Additionally, it discusses the efficiency of columns through theoretical plates and the selection criteria for stationary phases, being relevant for numerous applications in chemical and biological analysis.