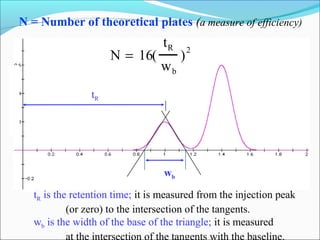
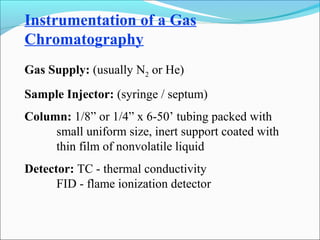
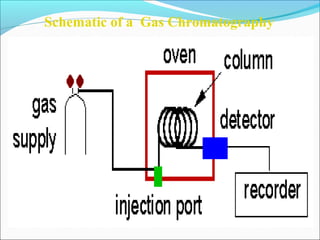
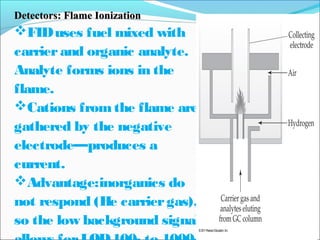
The document discusses chromatography, a technique invented by Mikhail Tswett in 1901 to separate plant pigments. It covers the workings of gas chromatography, its instrumentation, and applications, highlighting its efficiency in analyzing volatile samples and its role in pharmaceutical quality control. Various types of chromatography and detectors used are also described.