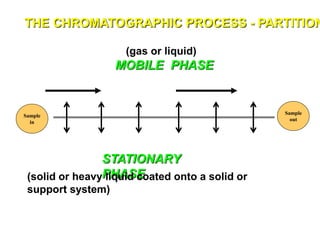
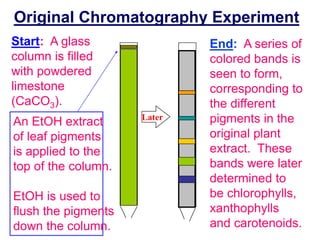
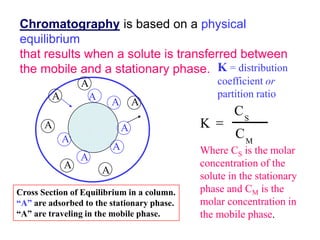
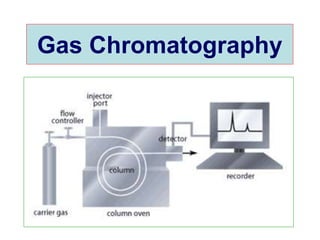
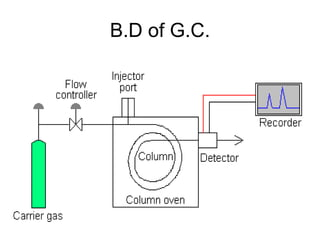
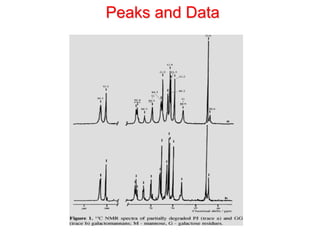
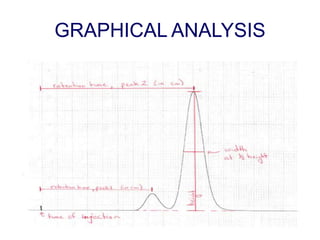
1. Gas chromatography is a technique used to separate mixtures by partitioning components between a stationary and mobile liquid or gas phase.
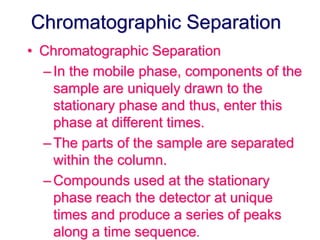
2. It works by forcing a gaseous or volatile sample mixture through a column containing a solid or liquid stationary phase, which interacts differently with each component. This causes the components to elute from the column at different times.
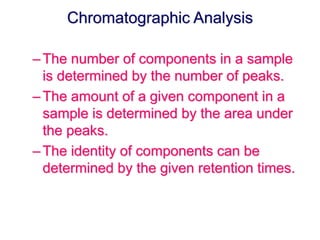
3. Gas chromatography is useful for separating volatile organic compounds and finds applications in fields like drug analysis and purity testing due to its high resolution, sensitivity, speed, and ability to perform both qualitative and quantitative analysis.