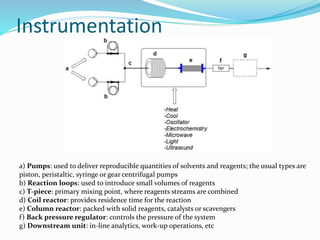
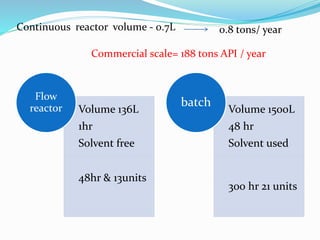
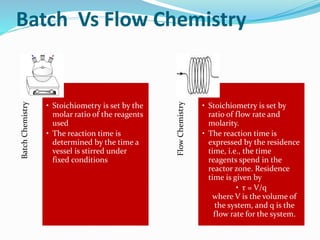
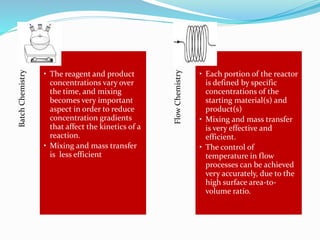
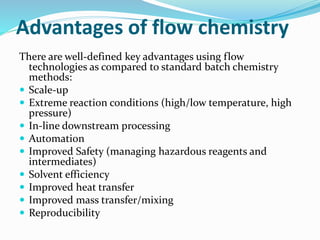
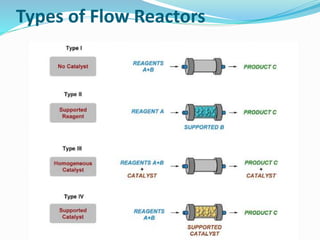
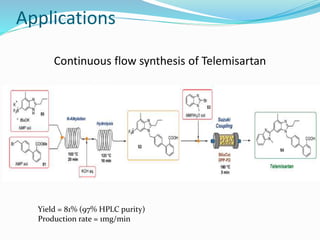
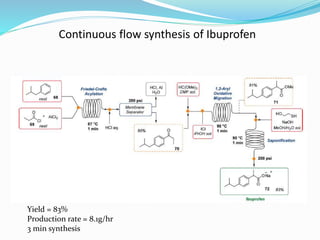
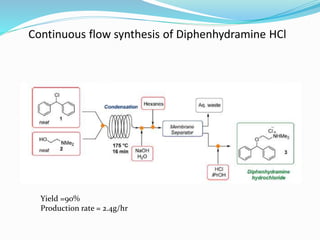
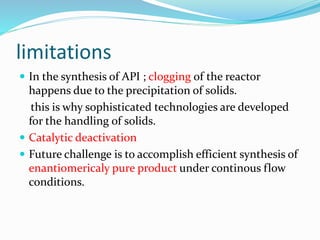
This document discusses flow chemistry and its advantages over traditional batch chemistry. Flow chemistry involves pumping reagents continuously through reactors to perform reactions. It offers benefits like improved safety, reproducibility, mixing, heat/mass transfer. Several types of flow reactors and applications in drug synthesis are described. Advantages include ease of scale-up, ability to achieve extreme reaction conditions, integration with downstream processing, and automation. Limitations include potential for clogging and catalytic deactivation over time.