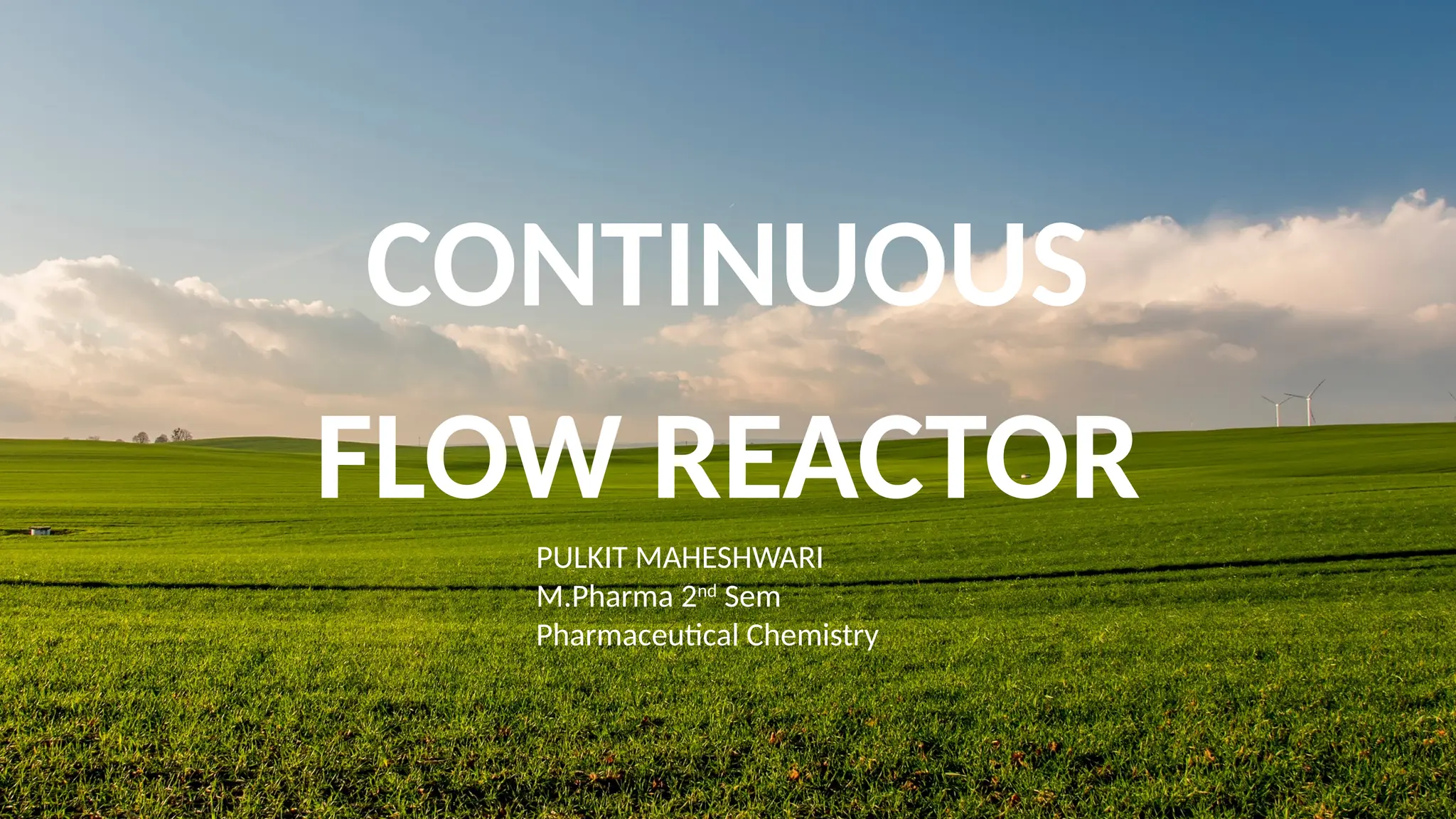
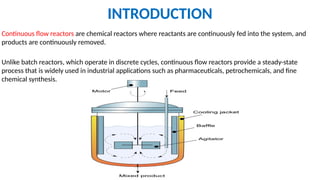
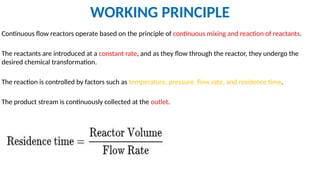
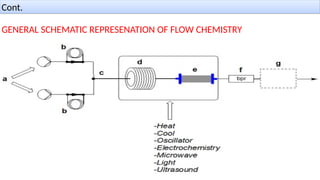
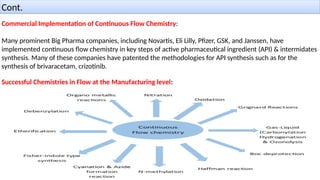
M.PHARMA CHEMISTRY 1st Year 2nd Semester ADVANCED ORGANIC CHEMISTRY - 2 (MPC202T) Unit - 1 Green Chemistry - d) Continuous flow reactors
Contents Include - Working principle , types , advantage , disadvantage , synthetic application , recent advances