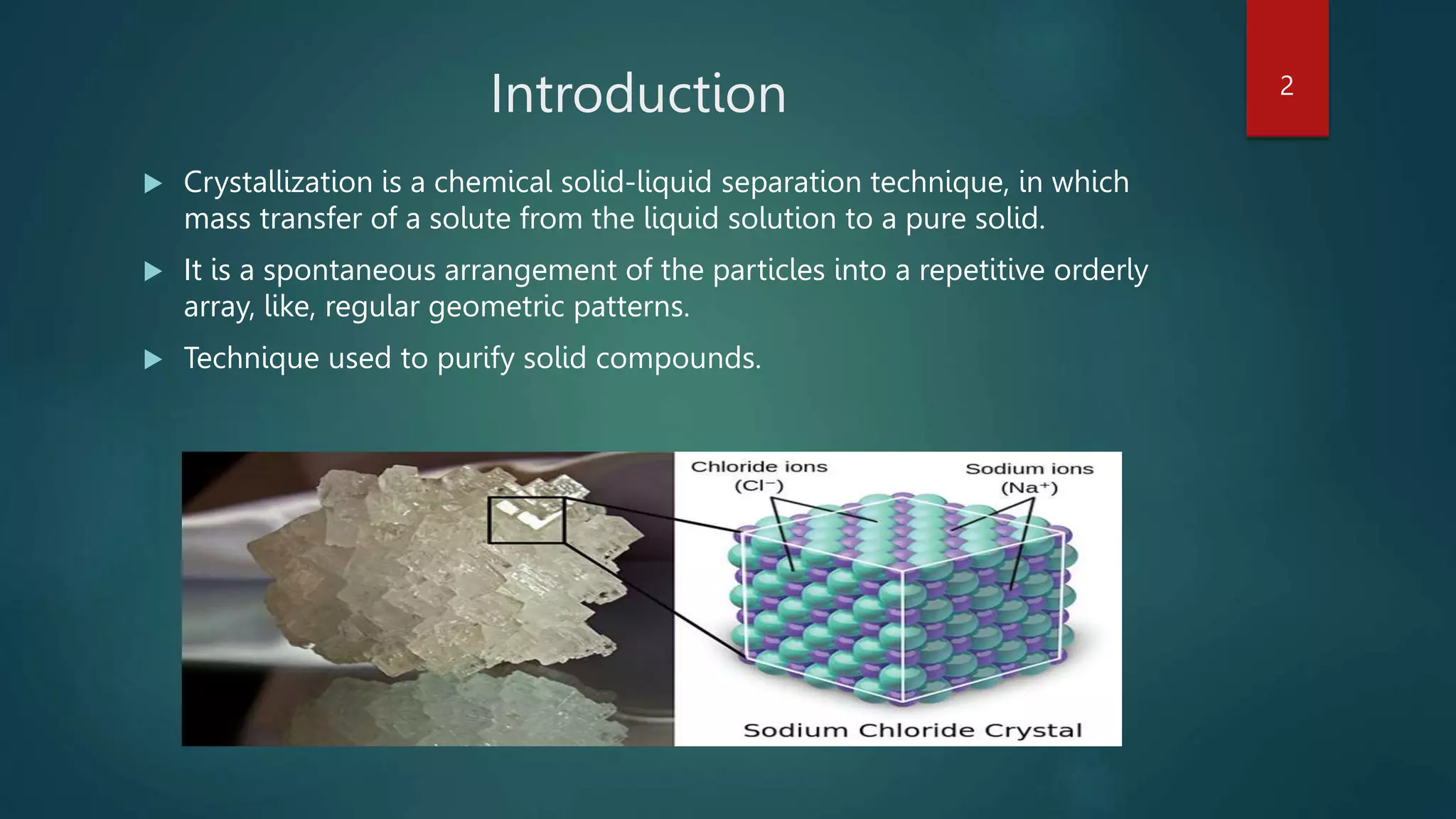
The document discusses crystallization as a solid-liquid separation technique, detailing methods from aqueous and non-aqueous solutions, and factors affecting crystallization. Key aspects include the role of water of crystallization, solvent effects, nucleation processes, and optimal conditions for crystal growth. It emphasizes the importance of time and environment in achieving high-quality crystal formation.