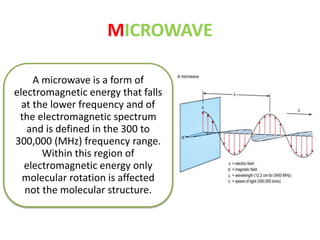
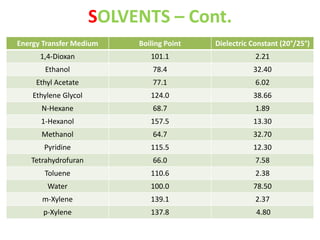
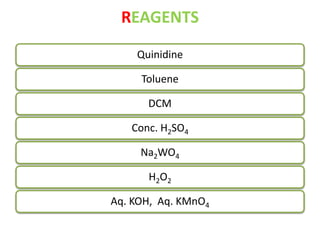
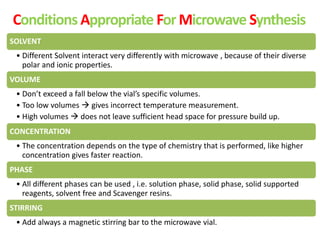
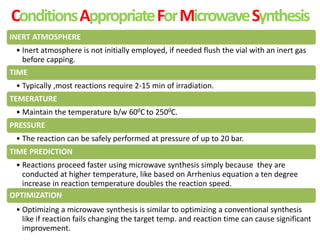
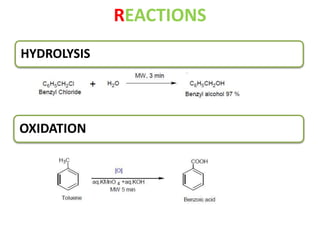
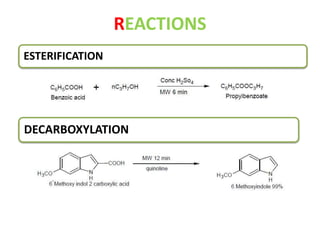
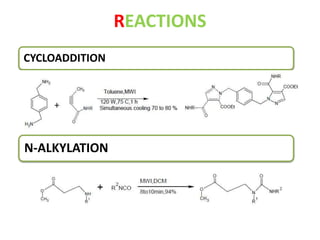
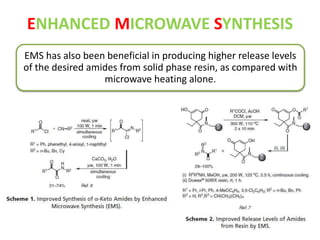
This document discusses microwave-assisted organic chemistry (MORE chemistry) as an eco-friendly technology. It provides advantages of MORE chemistry such as being easy, effective, and economic while requiring less solvents. The document then discusses how microwaves affect molecular rotation but not structure in organic molecules. It also outlines benefits of microwave-assisted organic synthesis like faster reactions, higher temperatures, and energy efficiency. Examples of reactions that can be conducted include hydrolysis, oxidation, esterification, and decarboxylation. In conclusion, the document discusses how MORE chemistry can improve industrial organic synthesis in a cost-effective and environmentally-friendly manner.