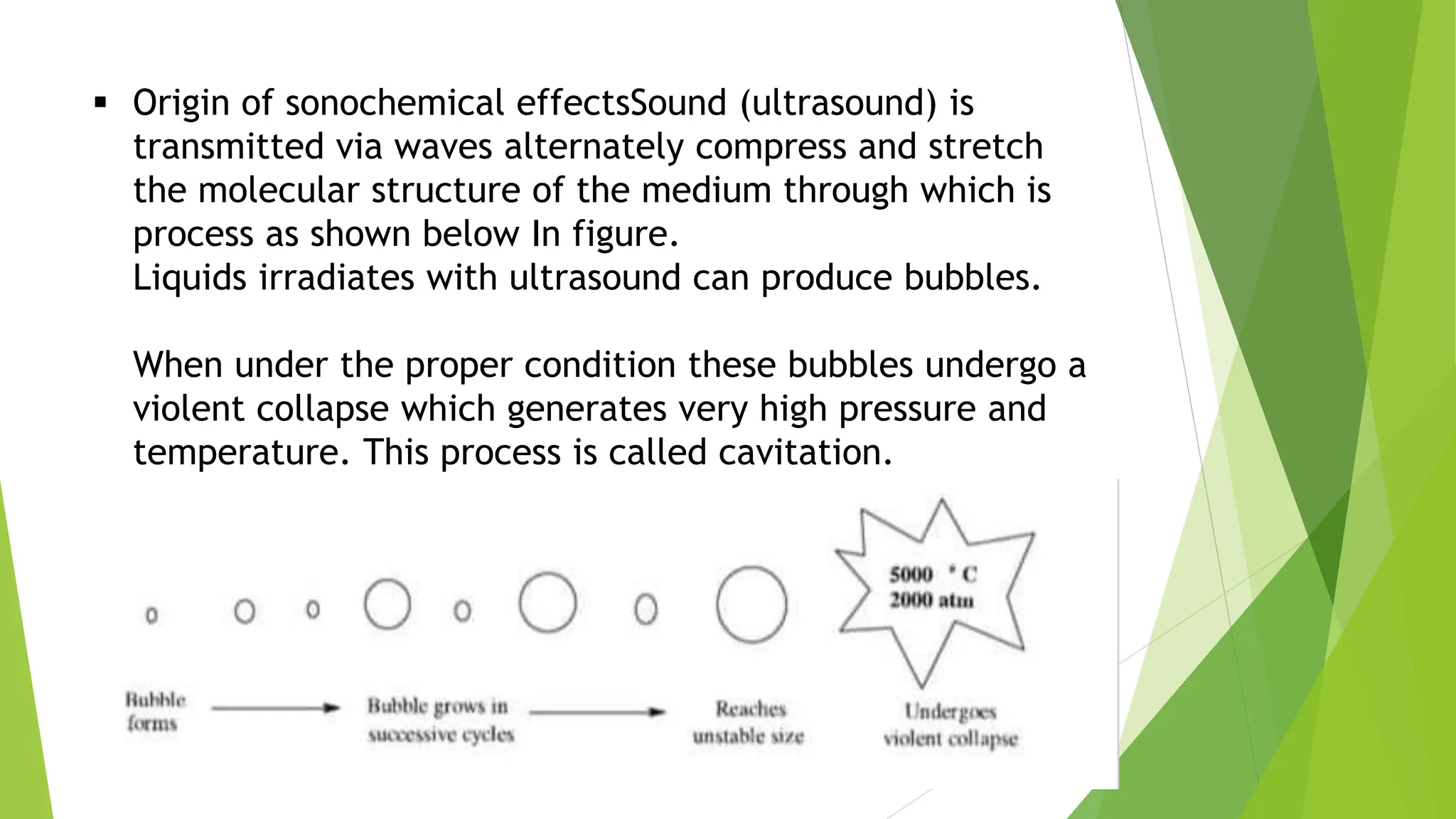
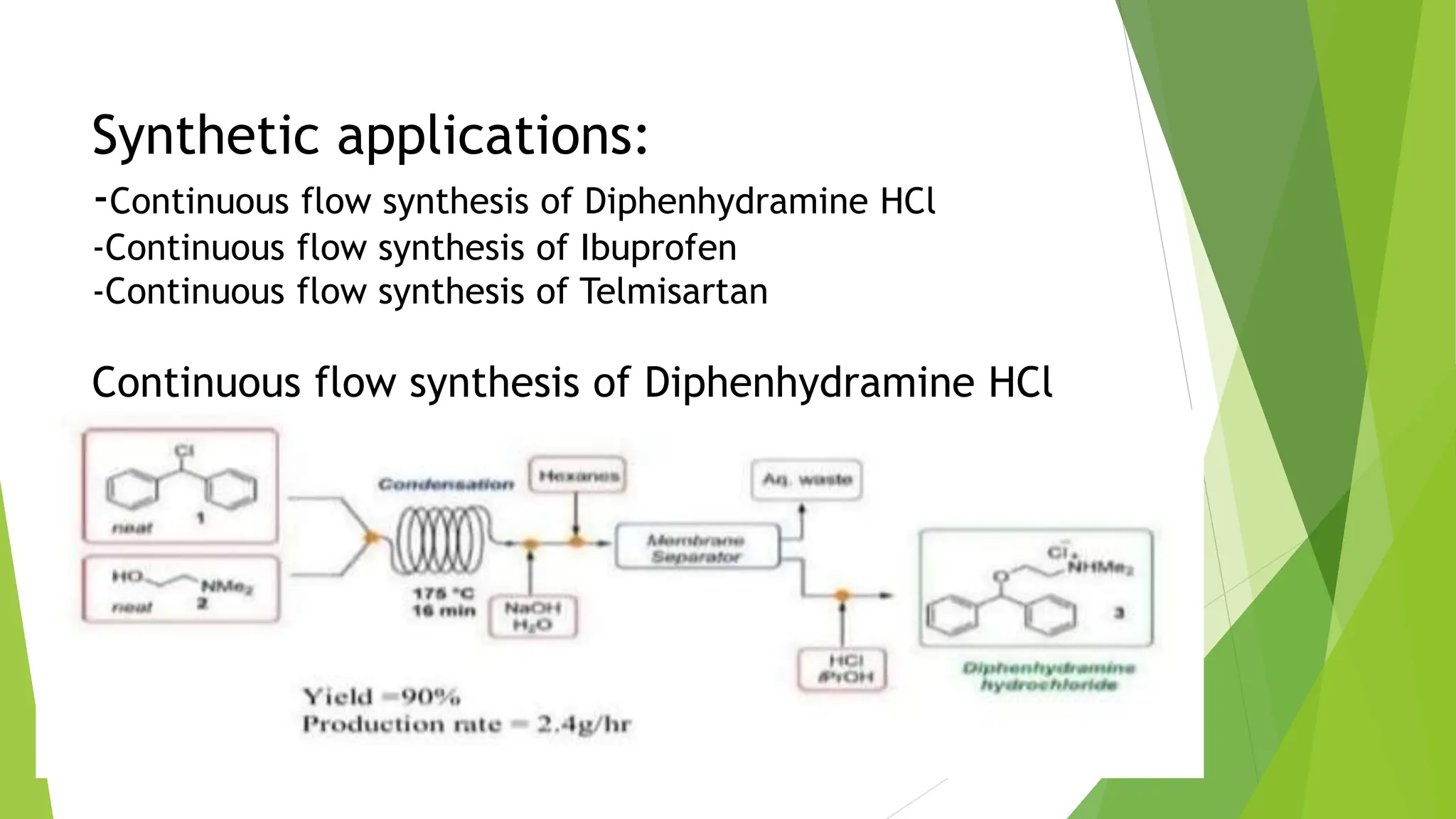
The document discusses ultrasound-assisted reactions and continuous flow reactors, focusing on the principles, types of sonochemical reactions, and their synthetic applications. It explains the mechanism of sonochemistry, including cavitation and its effects on chemical reactivity, as well as the advantages of continuous flow reactors such as improved safety, efficiency, and scalability. The document highlights specific synthetic applications and medical and industrial uses of ultrasound technology.