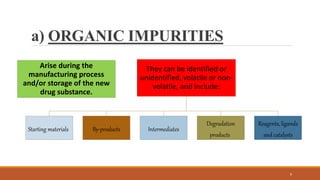
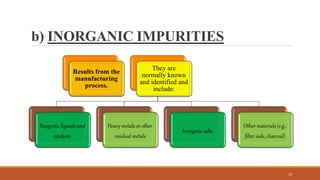
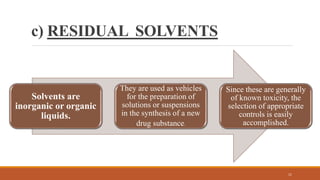
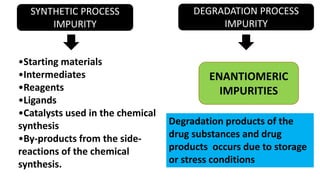
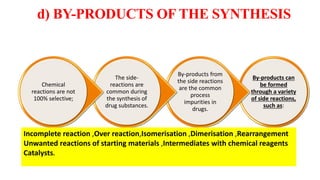
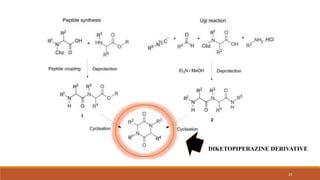
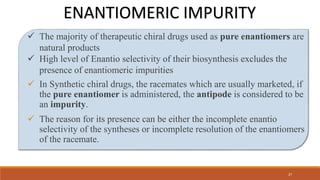
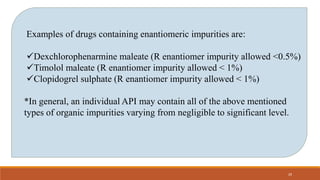
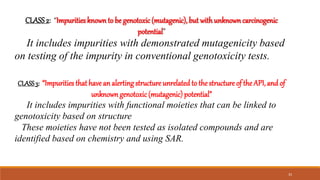
The document discusses various types of impurities that can be present in pharmaceutical products, including organic, inorganic, and residual solvent impurities. It describes potential sources of impurities such as starting materials, reagents, catalysts, and degradation processes. It also discusses genotoxic impurities and establishes a five-class system for categorizing them based on their known or suspected toxicity and carcinogenic properties.