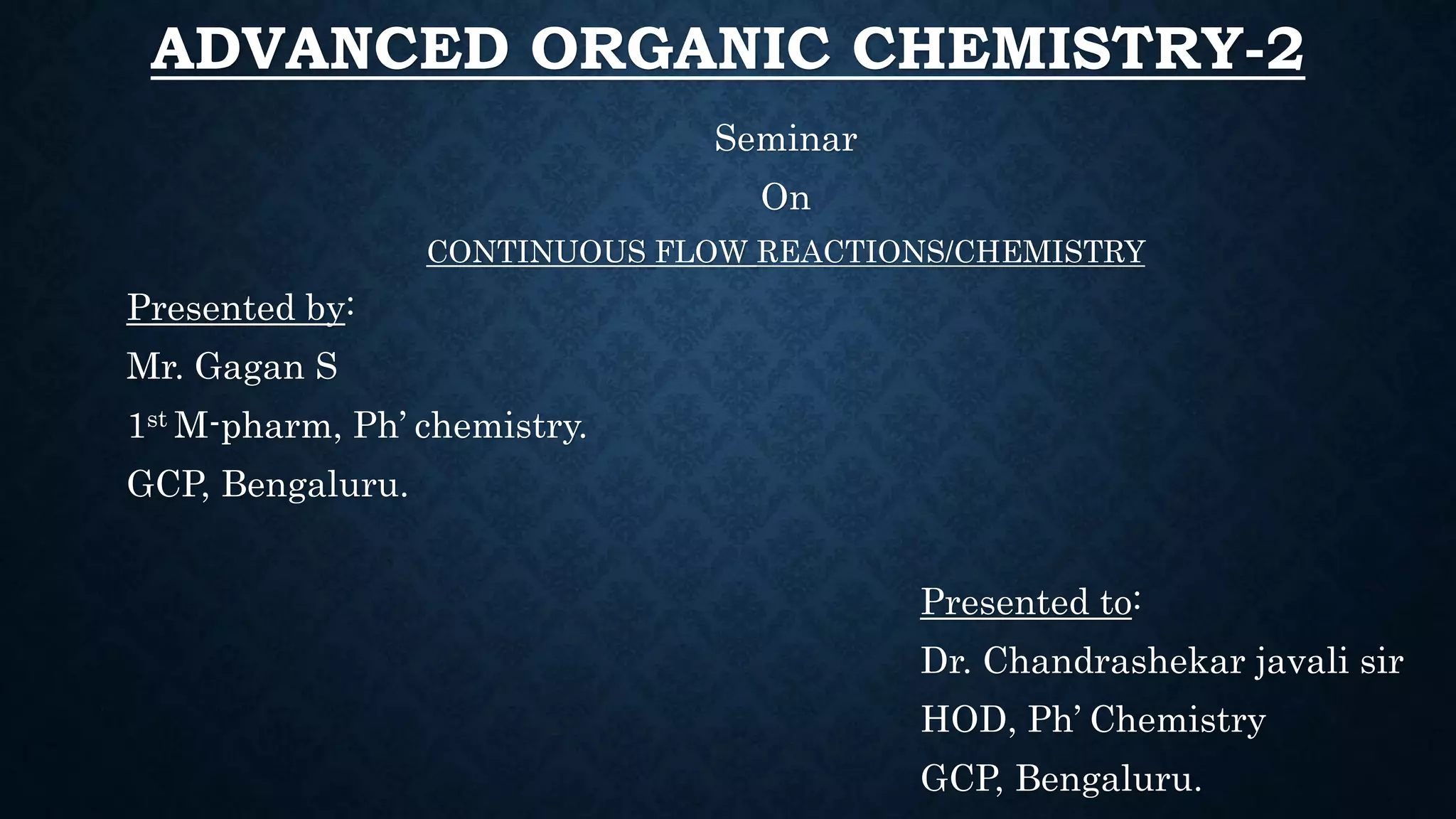
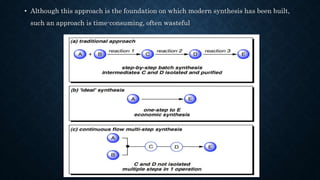
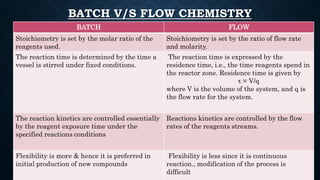
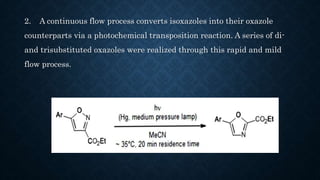
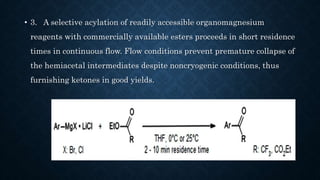
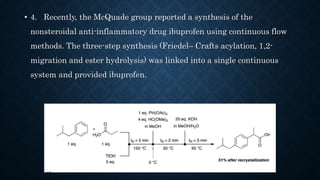
This document presents an advanced seminar on continuous flow reactions, highlighting the differences between batch and flow chemistry, including their advantages and disadvantages. Continuous flow techniques streamline multi-step syntheses by combining reactions without the need for isolating intermediates, offering improved efficiency for producing complex compounds. Various types of reactors and their specific applications, as well as notable synthetic examples such as the synthesis of ibuprofen, are discussed.