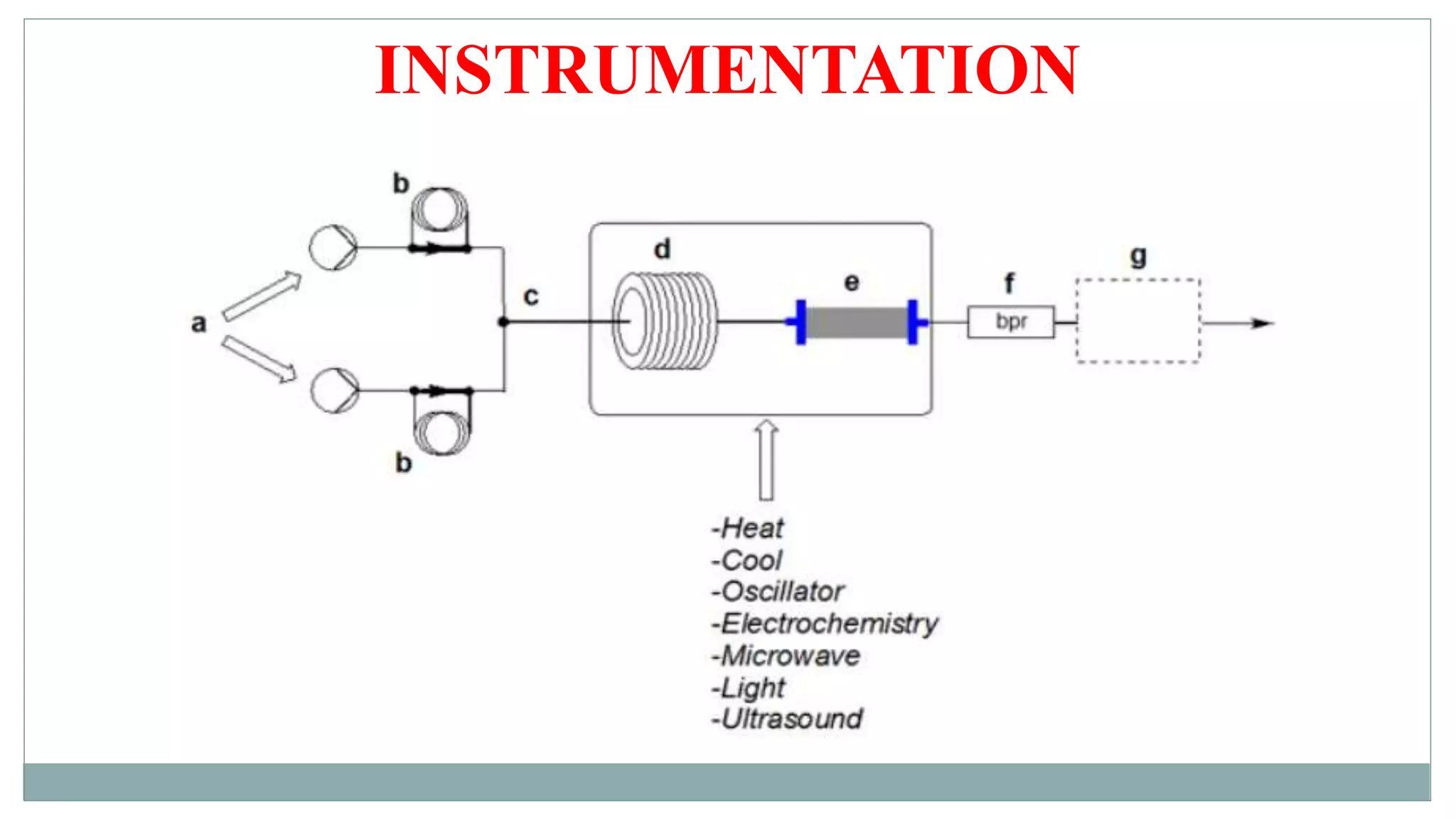
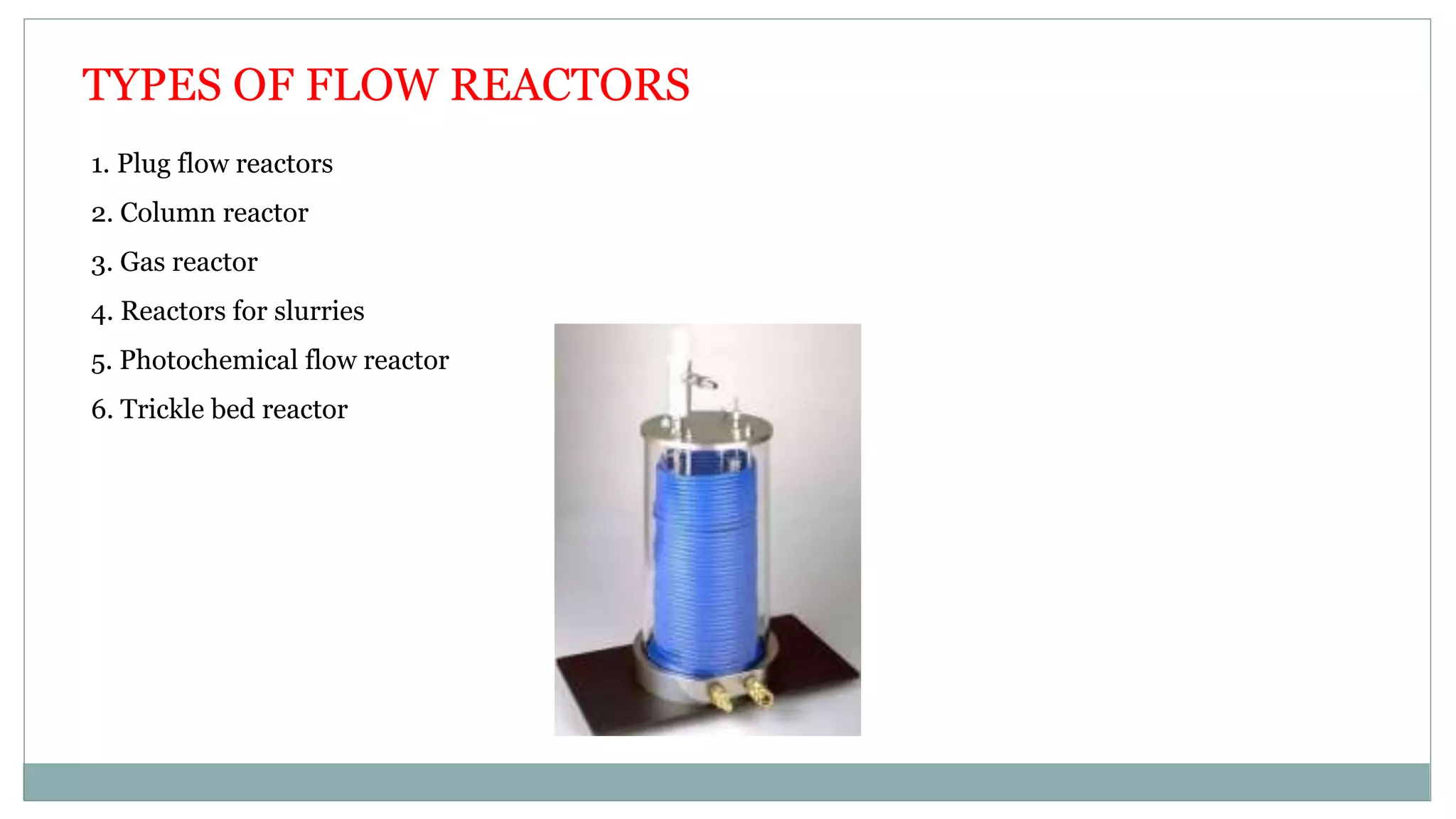
The document discusses continuous flow reactors for chemical synthesis. It introduces continuous flow chemistry as pumping reactants at controlled flow rates through reactors. This allows reactions to occur safely under extreme conditions with high product quality and reproducibility. The key components of flow reactors are described including pumps, mixing points, coils, and columns. Parameters like residence time, flow rates and stoichiometry are also discussed. Finally, examples of synthetic applications for continuous flow reactors producing pharmaceutical compounds are provided.