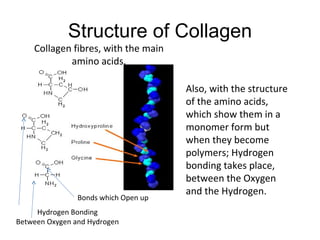
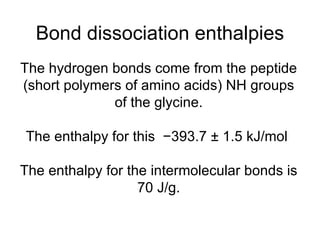
Collagen is a fibrous protein found in connective tissues like bones, skin, and organs. It provides structure, strength, and firmness to tissues. As we age, collagen levels decrease, causing wrinkles and joint weakness. Collagen's structure involves hydrogen bonding between amino acids that forms collagen fibers. This hydrogen bonding gives collagen its strength and suitability for uses in tendons, bones, and skin.